Chelating Agent Functionalized Substrates for the Formation of Thick Films via Electrophoretic Deposition
- PMID: 34222203
- PMCID: PMC8245681
- DOI: 10.3389/fchem.2021.703528
Chelating Agent Functionalized Substrates for the Formation of Thick Films via Electrophoretic Deposition
Abstract
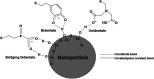
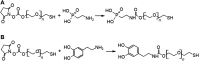
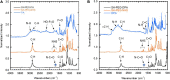
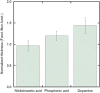
Incorporating nanoparticles into devices for a wide range of applications often requires the formation of thick films, which is particularly necessary for improving magnetic power storage, microwave properties, and sensor performance. One approach to assembling nanoparticles into films is the use of electrophoretic deposition (EPD). This work seeks to develop methods to increase film thickness and stability in EPD by increasing film-substrate interactions via functionalizing conductive substrates with various chelating agents. Here, we deposited iron oxide nanoparticles onto conductive substrates functionalized with three chelating agents with different functional moieties and differing chelating strengths. We show that increasing chelating strength can increase film-substrate interactions, resulting in thicker films when compared to traditional EPD. Results will also be presented on how the chelating strength relates to film formation as a function of deposition conditions. Yield for EPD is influenced by deposition conditions including applied electric field, particle concentration, and deposition time. This work shows that the functionalization of substrates with chelating agents that coordinate strongly with nanoparticles (phosphonic acid and dopamine) overcome parameters that traditionally hinder the deposition of thicker and more stable films, such as applied electric field and high particle concentration. We show that functionalizing substrates with chelating agents is a promising method to fabricate thick, stable films of nanoparticles deposited via EPD over a larger processing space by increasing film-substrate interactions.
Keywords: assembly; chelating ligands; electrophorectic deposition; magnetic nanopartcles; nanomaterials.
Copyright © 2021 Mills, Starr, Bohannon and Andrew.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Amrollahi P., Krasinski J. S., Vaidyanathan R., Tayebi L., Vashaee D. (2015). Handbook of Nanoelectrochemistry. Switzerland: Springer International Publishing.
-
- ASTM (2017). Standard Test Methods for Rating Adhesion for Tape Test. ASTM D3359-17. West Conshohocken, PA: American Society for Testing Materials International.
-
- Besra L., Liu M. (2007). A Review on Fundamentals and Applications of Electrophoretic Deposition (EPD). Prog. Mater. Sci. 52, 1–61. 10.1016/j.pmatsci.2006.07.001 - DOI
-
- Bonvin D., Bastiaansen J. A. M., Stuber M., Hofmann H., Mionić Ebersold M. (2017). Chelating Agents as Coating Molecules for Iron Oxide Nanoparticles. RSC Adv. 7, 55598–55609. 10.1039/c7ra08217g - DOI
-
- Boyer C., Whittaker M. R., Bulmus V., Liu J., Davis T. P. (2010). The Design and Utility of Polymer-Stabilized Iron-Oxide Nanoparticles for Nanomedicine Applications. NPG Asia Mater. 2, 23–30. 10.1038/asiamat.2010.6 - DOI
LinkOut - more resources
Full Text Sources

