High-Efficient Production of Adipose-Derived Stem Cell (ADSC) Secretome Through Maturation Process and Its Non-scarring Wound Healing Applications
- PMID: 34222219
- PMCID: PMC8242583
- DOI: 10.3389/fbioe.2021.681501
High-Efficient Production of Adipose-Derived Stem Cell (ADSC) Secretome Through Maturation Process and Its Non-scarring Wound Healing Applications
Abstract
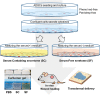
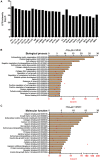
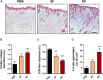
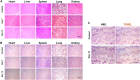
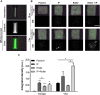
Recently, the stem cell-derived secretome, which is the set of proteins expressed by stem cells and secreted into the extracellular space, has been demonstrated as a critical contributor for tissue repair. In this study, we have produced two sets of high concentration secretomes from adipose-derived mesenchymal stem cells (ADSCs) that contain bovine serum or free of exogenous molecules. Through proteomic analysis, we elucidated that proteins related to extracellular matrix organization and growth factor-related proteins are highly secreted by ADSCs. Additionally, the application of ADSC secretome to full skin defect showed accelerated wound closure, enhanced angiogenic response, and complete regeneration of epithelial gaps. Furthermore, the ADSC secretome was capable of reducing scar formation. Finally, we show high-dose injection of ADSC secretome via intraperitoneal or transdermal delivery demonstrated no detectable pathological conditions in various tissues/organs, which supports the notion that ADSC secretome can be safely utilized for tissue repair and regeneration.
Keywords: proteomic analysis; secretome; skin regeneration; stem cells; tissue repair.
Copyright © 2021 An, Kim, Lee, Lee, Park, Ko, Kim, Koh, Hong, Son, Cho, Park, Kim and Hwang.
Conflict of interest statement
DHK, EL, JWK, and S-DK were employed by the company, Senior Science & Life, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- An Y. H., Park M. J., Lee J., Ko J., Kim S. H., Kang D. H., et al. (2020). Recent advances in the transdermal delivery of protein therapeutics with a combinatorial system of chemical adjuvants and physical penetration enhancements. Adv. Ther. Germany 3:1900116. 10.1002/adtp.201900116 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

