Current Applications and Future Directions of Bioengineering Approaches for Bladder Augmentation and Reconstruction
- PMID: 34222316
- PMCID: PMC8249581
- DOI: 10.3389/fsurg.2021.664404
Current Applications and Future Directions of Bioengineering Approaches for Bladder Augmentation and Reconstruction
Abstract
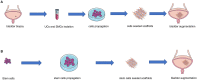
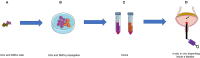
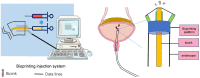
End-stage neurogenic bladder usually results in the insufficiency of upper urinary tract, requiring bladder augmentation with intestinal tissue. To avoid complications of augmentation cystoplasty, tissue-engineering technique could offer a new approach to bladder reconstruction. This work reviews the current state of bioengineering progress and barriers in bladder augmentation or reconstruction and proposes an innovative method to address the obstacles of bladder augmentation. The ideal tissue-engineered bladder has the characteristics of high biocompatibility, compliance, and specialized urothelium to protect the upper urinary tract and prevent extravasation of urine. Despite that many reports have demonstrated that bioengineered bladder possessed a similar structure to native bladder, few large animal experiments, and clinical applications have been performed successfully. The lack of satisfactory outcomes over the past decades may have become an important factor hindering the development in this field. More studies should be warranted to promote the use of tissue-engineered bladders in clinical practice.
Keywords: 3D bioprinting; bladder augmentation; bladder reconstruction; scaffolds; tissue engineering.
Copyright © 2021 Wang, Zhang and Liao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer PZ declared a shared affiliation, with no collaboration, with the authors to the handling editor at the time of the review.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

