Epidemiology of Trypanosomiasis in Wildlife-Implications for Humans at the Wildlife Interface in Africa
- PMID: 34222391
- PMCID: PMC8248802
- DOI: 10.3389/fvets.2021.621699
Epidemiology of Trypanosomiasis in Wildlife-Implications for Humans at the Wildlife Interface in Africa
Abstract
While both human and animal trypanosomiasis continue to present as major human and animal public health constraints globally, detailed analyses of trypanosome wildlife reservoir hosts remain sparse. African animal trypanosomiasis (AAT) affects both livestock and wildlife carrying a significant risk of spillover and cross-transmission of species and strains between populations. Increased human activity together with pressure on land resources is increasing wildlife-livestock-human infections. Increasing proximity between human settlements and grazing lands to wildlife reserves and game parks only serves to exacerbate zoonotic risk. Communities living and maintaining livestock on the fringes of wildlife-rich ecosystems require to have in place methods of vector control for prevention of AAT transmission and for the treatment of their livestock. Major Trypanosoma spp. include Trypanosoma brucei rhodesiense, Trypanosoma brucei gambiense, and Trypanosoma cruzi, pathogenic for humans, and Trypanosoma vivax, Trypanosoma congolense, Trypanosoma evansi, Trypanosoma brucei brucei, Trypanosoma dionisii, Trypanosoma thomasbancrofti, Trypanosma elephantis, Trypanosoma vegrandis, Trypanosoma copemani, Trypanosoma irwini, Trypanosoma copemani, Trypanosoma gilletti, Trypanosoma theileri, Trypanosoma godfreyi, Trypansoma simiae, and Trypanosoma (Megatrypanum) pestanai. Wildlife hosts for the trypansomatidae include subfamilies of Bovinae, Suidae, Pantherinae, Equidae, Alcephinae, Cercopithecinae, Crocodilinae, Pteropodidae, Peramelidae, Sigmodontidae, and Meliphagidae. Wildlife species are generally considered tolerant to trypanosome infection following centuries of coexistence of vectors and wildlife hosts. Tolerance is influenced by age, sex, species, and physiological condition and parasite challenge. Cyclic transmission through Glossina species occurs for T. congolense, T. simiae, T. vivax, T. brucei, and T. b. rhodesiense, T. b. gambiense, and within Reduviid bugs for T. cruzi. T. evansi is mechanically transmitted, and T. vixax is also commonly transmitted by biting flies including tsetse. Wildlife animal species serve as long-term reservoirs of infection, but the delicate acquired balance between trypanotolerance and trypanosome challenge can be disrupted by an increase in challenge and/or the introduction of new more virulent species into the ecosystem. There is a need to protect wildlife, animal, and human populations from the infectious consequences of encroachment to preserve and protect these populations. In this review, we explore the ecology and epidemiology of Trypanosoma spp. in wildlife.
Keywords: Trypanosoma brucei gambiense; Trypanosoma brucei rhodesiense; human African trypanosomiasis; human-wildlife interactions; sleeping sickness; trypanosomes in wildlife; wildlife-livestock interactions.
Copyright © 2021 Kasozi, Zirintunda, Ssempijja, Buyinza, Alzahrani, Matama, Nakimbugwe, Alkazmi, Onanyang, Bogere, Ochieng, Islam, Matovu, Nalumenya, Batiha, Osuwat, Abdelhamid, Shen, Omadang and Welburn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
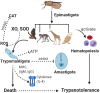
References
-
- World Health Organization . Tropical Disease Research: Progress 1975-94, Highlights 1993-94, Twelfth Programme Report of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). Geneva: World Health Organization; (1995). Available online at: https://www.who.int/tdr/publications/progress-75-94/en/
-
- Barbosa A, Reiss A, Jackson B, Warren K, Paparini A, Gillespie G, et al. Prevalence, genetic diversity and potential clinical impact of blood-borne and enteric protozoan parasites in native mammals from northern Australia. Vet Parasitol. (2017) 238:94–105. 10.1016/j.vetpar.2017.04.007 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

