The antiviral activity of iota-, kappa-, and lambda-carrageenan against COVID-19: A critical review
- PMID: 34222718
- PMCID: PMC8240443
- DOI: 10.1016/j.cegh.2021.100826
The antiviral activity of iota-, kappa-, and lambda-carrageenan against COVID-19: A critical review
Abstract
Objective: There is no specific antiviral treatment available for coronavirus disease 2019 (COVID-19). Among the possible natural constituents is carrageenan, a polymer derived from marine algae that possesses a variety of antiviral properties. The purpose of this review was to summarize the evidence supporting carrageenan subtypes' antiviral activity against the emerging severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the causative agent of COVID-19.
Methods: PubMed/MEDLINE and Google Scholar searches were conducted for publications using the terms 'carrageenan', 'iota carrageenan', 'kappa carrageenan', lambda-carrageenan', 'coronavirus', 'common cold', 'rhinovirus', and 'SARS-CoV-2' search was also done in grey literature to increase our understanding. A search for the word "carrageenan" was also carried out. Most of the publications were discussed in narrative.
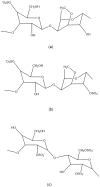
Results: Carrageenan has been shown to have potent antiviral activity against both coronaviruses (coronavirus NL63, SARS-CoV-2) and non-coronaviruses such as dengue virus, herpes simplex virus, cytomegalovirus, vaccinia virus, vesicular stomatitis virus, sindbis virus, human immunodeficiency virus, influenza virus, human papillomavirus, rabies virus, junin virus, tacaribe virus, African swine fever, bovine herpes virus, suid herpes virus, and rhinovirus. No in vivo study has been conducted using carrageenan as an anti-SARS-CoV-2 agent. The majority of the in vivo research was done on influenza, a respiratory virus that causes common cold together with coronavirus. Thus, various clinical trials were conducted to determine the transferability of these in vitro data to clinical effectiveness against SARS-CoV-2. When combined with oral ivermectin, nasally administered iota-carrageenan improved outcome in COVID-19 patients. It is still being tested in clinics for single-dose administration.
Conclusion: Though the carrageenan exhibited potent antiviral activity against SARS-CoV-2 and was used to treat COVID-19 under emergency protocol in conjunction with oral medications such as ivermectin, there is no solid evidence from clinical trials to support its efficacy. Thus, clinical trials are required to assess its efficacy for COVID-19 treatment prior to broad application.
Keywords: COVID-19; Carrageenan; Clinical trial; Iota-carrageenan; Nasal spray; SARS-CoV-2.
© 2021 The Author.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro.PLoS One. 2021 Feb 17;16(2):e0237480. doi: 10.1371/journal.pone.0237480. eCollection 2021. PLoS One. 2021. PMID: 33596218 Free PMC article.
-
The Saliva of Probands Sucking an Iota-Carrageenan Containing Lozenge Inhibits Viral Binding and Replication of the Most Predominant Common Cold Viruses and SARS-CoV-2.Int J Gen Med. 2021 Sep 7;14:5241-5249. doi: 10.2147/IJGM.S325861. eCollection 2021. Int J Gen Med. 2021. PMID: 34526804 Free PMC article. Clinical Trial.
-
Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta.Int J Mol Sci. 2021 Dec 8;22(24):13202. doi: 10.3390/ijms222413202. Int J Mol Sci. 2021. PMID: 34947999 Free PMC article.
-
Molnupiravir and Its Antiviral Activity Against COVID-19.Front Immunol. 2022 Apr 4;13:855496. doi: 10.3389/fimmu.2022.855496. eCollection 2022. Front Immunol. 2022. PMID: 35444647 Free PMC article. Review.
-
Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data.Trends Food Sci Technol. 2020 Oct;104:219-234. doi: 10.1016/j.tifs.2020.08.006. Epub 2020 Aug 19. Trends Food Sci Technol. 2020. PMID: 32836826 Free PMC article. Review.
Cited by
-
The Potential of Dietary Bioactive Compounds against SARS-CoV-2 and COVID-19-Induced Endothelial Dysfunction.Molecules. 2022 Mar 1;27(5):1623. doi: 10.3390/molecules27051623. Molecules. 2022. PMID: 35268723 Free PMC article. Review.
-
Polysaccharides and Their Derivatives as Potential Antiviral Molecules.Viruses. 2022 Feb 18;14(2):426. doi: 10.3390/v14020426. Viruses. 2022. PMID: 35216019 Free PMC article. Review.
-
Bioactive Carbohydrate Polymers-Between Myth and Reality.Molecules. 2021 Nov 23;26(23):7068. doi: 10.3390/molecules26237068. Molecules. 2021. PMID: 34885655 Free PMC article. Review.
-
Antiviral Activity of Carrageenans and Processing Implications.Mar Drugs. 2021 Jul 30;19(8):437. doi: 10.3390/md19080437. Mar Drugs. 2021. PMID: 34436276 Free PMC article. Review.
-
Synergistic Antiviral Activity of Xanthan Gum and Camostat Against Influenza Virus Infection.Viruses. 2025 Feb 21;17(3):301. doi: 10.3390/v17030301. Viruses. 2025. PMID: 40143232 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
