The role of the medial prefrontal cortex in cognition, ageing and dementia
- PMID: 34222873
- PMCID: PMC8249104
- DOI: 10.1093/braincomms/fcab125
The role of the medial prefrontal cortex in cognition, ageing and dementia
Abstract
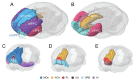
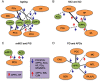
Humans require a plethora of higher cognitive skills to perform executive functions, such as reasoning, planning, language and social interactions, which are regulated predominantly by the prefrontal cortex. The prefrontal cortex comprises the lateral, medial and orbitofrontal regions. In higher primates, the lateral prefrontal cortex is further separated into the respective dorsal and ventral subregions. However, all these regions have variably been implicated in several fronto-subcortical circuits. Dysfunction of these circuits has been highlighted in vascular and other neurocognitive disorders. Recent advances suggest the medial prefrontal cortex plays an important regulatory role in numerous cognitive functions, including attention, inhibitory control, habit formation and working, spatial or long-term memory. The medial prefrontal cortex appears highly interconnected with subcortical regions (thalamus, amygdala and hippocampus) and exerts top-down executive control over various cognitive domains and stimuli. Much of our knowledge comes from rodent models using precise lesions and electrophysiology readouts from specific medial prefrontal cortex locations. Although, anatomical disparities of the rodent medial prefrontal cortex compared to the primate homologue are apparent, current rodent models have effectively implicated the medial prefrontal cortex as a neural substrate of cognitive decline within ageing and dementia. Human brain connectivity-based neuroimaging has demonstrated that large-scale medial prefrontal cortex networks, such as the default mode network, are equally important for cognition. However, there is little consensus on how medial prefrontal cortex functional connectivity specifically changes during brain pathological states. In context with previous work in rodents and non-human primates, we attempt to convey a consensus on the current understanding of the role of predominantly the medial prefrontal cortex and its functional connectivity measured by resting-state functional MRI in ageing associated disorders, including prodromal dementia states, Alzheimer's disease, post-ischaemic stroke, Parkinsonism and frontotemporal dementia. Previous cross-sectional studies suggest that medial prefrontal cortex functional connectivity abnormalities are consistently found in the default mode network across both ageing and neurocognitive disorders such as Alzheimer's disease and vascular cognitive impairment. Distinct disease-specific patterns of medial prefrontal cortex functional connectivity alterations within specific large-scale networks appear to consistently feature in the default mode network, whilst detrimental connectivity alterations are associated with cognitive impairments independently from structural pathological aberrations, such as grey matter atrophy. These disease-specific patterns of medial prefrontal cortex functional connectivity also precede structural pathological changes and may be driven by ageing-related vascular mechanisms. The default mode network supports utility as a potential biomarker and therapeutic target for dementia-associated conditions. Yet, these associations still require validation in longitudinal studies using larger sample sizes.
Keywords: ageing; default mode network; dementia; prefrontal cortex; vascular cognitive impairment.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Wood JN, Grafman J.. Human prefrontal cortex: Processing and representational perspectives. Nat Rev Neurosci. 2003;4(2):139–147. - PubMed
-
- Tekin S, Cummings JL.. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. J Psychosom Res. 2002;53(2):647–654. - PubMed
-
- Rose JE, Woolsey CN.. The orbitofrontal cortex and its connections with the mediodorsal nucleus in rabbit, sheep and cat. Res Publ Assoc Res Nerv Ment Dis. 1948;27(1 vol):210–232. - PubMed
-
- Rose JE, Woolsey CN.. Structure and relations of limbic cortex and anterior thalamic nuclei in rabbit and cat. J Comp Neurol. 1948;89(3):279–347. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
