Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination
- PMID: 34223402
- PMCID: PMC8240500
- DOI: 10.1016/j.medj.2021.03.014
Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination
Abstract
Background: Development of an effective vaccine against the pathogenic blood-stage infection of human malaria has proved challenging, and no candidate vaccine has affected blood-stage parasitemia following controlled human malaria infection (CHMI) with blood-stage Plasmodium falciparum.
Methods: We undertook a phase I/IIa clinical trial in healthy adults in the United Kingdom of the RH5.1 recombinant protein vaccine, targeting the P. falciparum reticulocyte-binding protein homolog 5 (RH5), formulated in AS01B adjuvant. We assessed safety, immunogenicity, and efficacy against blood-stage CHMI. Trial registered at ClinicalTrials.gov, NCT02927145.
Findings: The RH5.1/AS01B formulation was administered using a range of RH5.1 protein vaccine doses (2, 10, and 50 μg) and was found to be safe and well tolerated. A regimen using a delayed and fractional third dose, in contrast to three doses given at monthly intervals, led to significantly improved antibody response longevity over ∼2 years of follow-up. Following primary and secondary CHMI of vaccinees with blood-stage P. falciparum, a significant reduction in parasite growth rate was observed, defining a milestone for the blood-stage malaria vaccine field. We show that growth inhibition activity measured in vitro using purified immunoglobulin G (IgG) antibody strongly correlates with in vivo reduction of the parasite growth rate and also identify other antibody feature sets by systems serology, including the plasma anti-RH5 IgA1 response, that are associated with challenge outcome.
Conclusions: Our data provide a new framework to guide rational design and delivery of next-generation vaccines to protect against malaria disease.
Funding: This study was supported by USAID, UK MRC, Wellcome Trust, NIAID, and the NIHR Oxford-BRC.
Keywords: CHMI; Plasmodium falciparum; RH5; blood-stage; clinical trial; malaria; systems serology; vaccine.
© 2021 The Author(s).
Conflict of interest statement
A.D.D. and S.J.D. are named inventors on patent applications relating to RH5 and/or other malaria vaccines and immunization regimens. W.A.d.J. is an employee of and shareholder in ExpreS2ion Biotechnologies, which has developed and is marketing the ExpreS2 cell expression platform. A.R.N. is an employee of Leidos, Inc., which holds the MVDP prime contract (AID-OAA-C-15-00071). A.M.M. has an immediate family member who is an inventor on patents relating to RH5 and/or other malaria vaccines and immunization regimens.
Figures
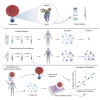

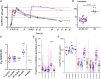
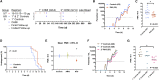

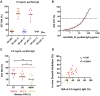

Comment in
-
Malaria vaccine trial shows the potentiating power of incremental progress.Med. 2021 Jun 11;2(6):635-636. doi: 10.1016/j.medj.2021.05.004. Epub 2021 Jun 15. Med. 2021. PMID: 35590135
References
-
- World Health Organization . 2019. World Malaria Report (2019)https://www.who.int/publications/i/item/9789241565721
-
- Moorthy V.S., Newman R.D., Okwo-Bele J.M. Malaria vaccine technology roadmap. Lancet. 2013;382:1700–1701. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

