Expanding the clinical phenotype in patients with disease causing variants associated with atypical Usher syndrome
- PMID: 34223797
- PMCID: PMC9233901
- DOI: 10.1080/13816810.2021.1946704
Expanding the clinical phenotype in patients with disease causing variants associated with atypical Usher syndrome
Abstract
Atypical Usher syndrome (USH) is poorly defined with a broad clinical spectrum. Here, we characterize the clinical phenotype of disease caused by variants in CEP78, CEP250, ARSG, and ABHD12.Chart review evaluating demographic, clinical, imaging, and genetic findings of 19 patients from 18 families with a clinical diagnosis of retinal disease and confirmed disease-causing variants in CEP78, CEP250, ARSG, or ABHD12.CEP78-related disease included sensorineural hearing loss (SNHL) in 6/7 patients and demonstrated a broad phenotypic spectrum including: vascular attenuation, pallor of the optic disc, intraretinal pigment, retinal pigment epithelium mottling, areas of mid-peripheral hypo-autofluorescence, outer retinal atrophy, mild pigmentary changes in the macula, foveal hypo-autofluorescence, and granularity of the ellipsoid zone. Nonsense and frameshift variants in CEP250 showed mild retinal disease with progressive, non-congenital SNHL. ARSG variants resulted in a characteristic pericentral pattern of hypo-autofluorescence with one patient reporting non-congenital SNHL. ABHD12-related disease showed rod-cone dystrophy with macular involvement, early and severe decreased best corrected visual acuity, and non-congenital SNHL ranging from unreported to severe.This study serves to expand the clinical phenotypes of atypical USH. Given the variable findings, atypical USH should be considered in patients with peripheral and macular retinal disease even without the typical RP phenotype especially when SNHL is noted. Additionally, genetic screening may be useful in patients who have clinical symptoms and retinal findings even in the absence of known SNHL given the variability of atypical USH.
Keywords: ABHD12; ARSG; Atypical usher syndrome; CEP78; cep250.
Conflict of interest statement
Figures
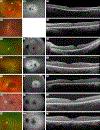


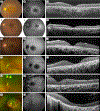
References
-
- Nolen RM, Hufnagel RB, Friedman TB, Turriff AE, Brewer CC, Zalewski CK, et al. Atypical and ultra-rare Usher syndrome: a patients with Usher syndrome type 1. J Hum Genet. 2010;55(12):796–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY024091/EY/NEI NIH HHS/United States
- R21 AG050437/AG/NIA NIH HHS/United States
- R01 EY018213/EY/NEI NIH HHS/United States
- U01 EY030580/EY/NEI NIH HHS/United States
- R01 EY009076/EY/NEI NIH HHS/United States
- R01 EY026682/EY/NEI NIH HHS/United States
- R24 EY027285/EY/NEI NIH HHS/United States
- P30 CA013696/CA/NCI NIH HHS/United States
- R24 EY028758/EY/NEI NIH HHS/United States
- K08 EY026650/EY/NEI NIH HHS/United States
- P30 EY010572/EY/NEI NIH HHS/United States
- U54 OD020351/OD/NIH HHS/United States
- R01 EY024698/EY/NEI NIH HHS/United States
- P30 EY019007/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
