Appearance of peanut agglutinin in the blood circulation after peanut ingestion promotes endothelial secretion of metastasis-promoting cytokines
- PMID: 34223877
- PMCID: PMC8643467
- DOI: 10.1093/carcin/bgab059
Appearance of peanut agglutinin in the blood circulation after peanut ingestion promotes endothelial secretion of metastasis-promoting cytokines
Abstract
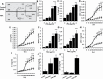
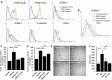
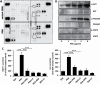
Peanut agglutinin (PNA) is a carbohydrate-binding protein in peanuts that accounts for ~0.15% peanut weight. PNA is highly resistant to cooking and digestion and is rapidly detectable in the blood after peanut consumption. Our previous studies have shown that circulating PNA mimics the actions of endogenous galactoside-binding protein galectin-3 by interaction with tumour cell-associated MUC1 and promotes circulating tumour cell metastatic spreading. The present study shows that circulating PNA interacts with micro- as well as macro-vascular endothelial cells and induces endothelial secretion of cytokines MCP-1 (CCL2) and IL-6 in vitro and in vivo. The increased secretion of these cytokines autocrinely/paracrinely enhances the expression of endothelial cell surface adhesion molecules including integrins, VCAM and selectin, leading to increased tumour cell-endothelial adhesion and endothelial tubule formation. Binding of PNA to endothelial surface MCAM (CD146), via N-linked glycans, and subsequent activation of PI3K-AKT-PREAS40 signalling is here shown responsible for PNA-induced secretion of MCP-1 and IL-6 by vascular endothelium. Thus, in addition to its influence on promoting tumour cell spreading by interaction with tumour cell-associated MUC1, circulating PNA might also influence metastasis by enhancing the secretion of metastasis-promoting MCP-1 and IL-6 from the vascular endothelium.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Ryder, S.D. et al. (1994) Peanut lectin stimulates proliferation in colonic explants from patients with inflammatory bowel disease and colon polyps. Gastroenterology, 106, 117–124. - PubMed
-
- Ryder, S.D. et al. (1992) Peanut lectin: a mitogen for normal human colonic epithelium and human HT29 colorectal cancer cells. J. Natl. Cancer Inst., 84, 1410–1416. - PubMed
-
- Wang, Q. et al. (1998) Identification of intact peanut lectin in peripheral venous blood. Lancet, 352, 1831–1832. - PubMed
-
- Yu, L.G. (2007) The oncofetal Thomsen-Friedenreich carbohydrate antigen in cancer progression. Glycoconj. J., 24, 411–420. - PubMed
-
- Yu, L.G. et al. (2007) Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J. Biol. Chem., 282, 773–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

