Development and Psychometric Validation of a Patient-Reported Outcome Measure for Arm Lymphedema: The LYMPH-Q Upper Extremity Module
- PMID: 34224044
- PMCID: PMC8349351
- DOI: 10.1245/s10434-021-09887-y
Development and Psychometric Validation of a Patient-Reported Outcome Measure for Arm Lymphedema: The LYMPH-Q Upper Extremity Module
Abstract
Background: A multiphased mixed-methods study was performed to develop and validate a comprehensive patient-reported outcome measure (PROM) for arm lymphedema in women with breast cancer (i.e., the LYMPH-Q Upper Extremity Module).
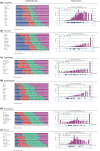
Methods: Qualitative interviews (January 2017 and June 2018) were performed with 15 women to elicit concepts specific to arm lymphedema after breast cancer treatment. Data were audio-recorded, transcribed, and coded. Scales were refined through cognitive interviews (October and Decemeber 2018) with 16 patients and input from 12 clinical experts. The scales were field-tested (October 2019 and January 2020) with an international sample of 3222 women in the United States and Denmark. Rasch measurement theory (RMT) analysis was used to examine reliability and validity.
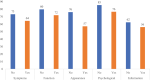
Results: The qualitative phase resulted in six independently functioning scales that measure arm symptoms, function, appearance, psychological function, and satisfaction with information and with arm sleeves. In the RMT analysis, all items in each scale had ordered thresholds and nonsignificant chi-square p values. For all the scales, the reliability statistics with and without extremes for the Person Separation Index were 0.80 or higher, Cronbach's alpha was 0.89 or higher, and the Intraclass Correlation Coefficients were 0.92 or higher. Lower (worse) scores on the LYMPH-Q Upper Extremity scales were associated with reporting of more severe arm swelling, an arm problem caused by cancer and/or its treatment, and wearing of an arm sleeve in the past 12 months.
Conclusions: The LYMPH-Q Upper Extremity Module can be used to measure outcomes that matter to women with upper extremity lymphedema. This new PROM was designed using a modern psychometric approach and, as such, can be used in research and in clinical care.
© 2021. The Author(s).
Conflict of interest statement
The LYMPH-Q Upper Extremity Module is owned by Memorial Sloan-Kettering Cancer Center, McMaster University, and Mass General Brigham, and Pusic and Klassen are co-developers. The remaining authors have no conflicts of interest.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

