A review of neuroradiological abnormalities in patients with coronavirus disease 2019 (COVID-19)
- PMID: 34224248
- PMCID: PMC8819585
- DOI: 10.1177/19714009211029177
A review of neuroradiological abnormalities in patients with coronavirus disease 2019 (COVID-19)
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to various neurological manifestations. There is an urgent need for a summary of neuroimaging findings to accelerate diagnosis and treatment plans. We reviewed prospective and retrospective studies to classify neurological abnormalities observed in patients with the SARS-CoV-2 infection.
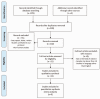
Methods: The relevant studies published in Scopus, PubMed and Clarivate Analytics databases were analysed. The search was performed for full-text articles published from 23 January 2020 to 23 February 2021.
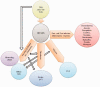
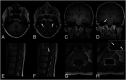
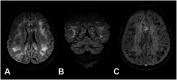
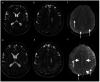
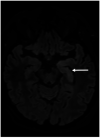
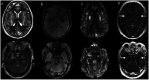
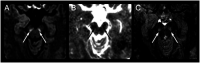
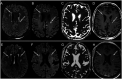
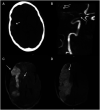
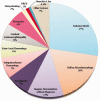
Results: In 23 studies the number of patients with SARS-CoV-2 infection was 20,850 and the number of patients with neurological manifestations was 1996 (9.5%). The total number of patients with neuroradiological abnormalities was 602 (2.8%). SARS-CoV-2 has led to various neuroimaging abnormalities which can be categorised by neuroanatomical localisation of lesions and their main probable underlying pathogenesis. Cranial nerve and spinal root abnormalities were cranial neuritis and polyradiculitis. Parenchymal abnormalities fell into four groups of: (a) thrombosis disorders, namely ischaemic stroke and sinus venous thrombosis; (b) endothelial dysfunction and damage disorders manifested as various types of intracranial haemorrhage and posterior reversible encephalopathy syndrome; (c) hypoxia/hypoperfusion disorders of leukoencephalopathy and watershed infarction; and (d) inflammatory disorders encompassing demyelinating disorders, encephalitis, vasculitis-like disorders, vasculopathy and cytotoxic lesions of the corpus callosum. Leptomeninges disorders included meningitis. Ischaemic stroke was the most frequent abnormality in these studies.
Conclusion: The review study suggests that an anatomical approach to the classification of heterogeneous neuroimaging findings in patients with SARS-CoV-2 and neurological manifestations would lend itself well for use by practitioners in diagnosis and treatment planning.
Keywords: COVID-19; Neuroradiological; brain; computed tomography; magnetic resonance; spinal.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

