The RNA editing enzyme ADAR2 restricts L1 mobility
- PMID: 34224323
- PMCID: PMC8677026
- DOI: 10.1080/15476286.2021.1940020
The RNA editing enzyme ADAR2 restricts L1 mobility
Abstract
Adenosine deaminases acting on RNA (ADARs) are enzymes that convert adenosines to inosines in double-stranded RNAs (RNA editing A-to-I). ADAR1 and ADAR2 were previously reported as HIV-1 proviral factors. The aim of this study was to investigate the composition of the ADAR2 ribonucleoprotein complex during HIV-1 expression. By using a dual-tag affinity purification procedure in cells expressing HIV-1 followed by mass spectrometry analysis, we identified 10 non-ribosomal ADAR2-interacting factors. A significant fraction of these proteins was previously found associated to the Long INterspersed Element 1 (LINE1 or L1) ribonucleoparticles and to regulate the life cycle of L1 retrotransposons. Considering that we previously demonstrated that ADAR1 is an inhibitor of LINE-1 retrotransposon activity, we investigated whether also ADAR2 played a similar function. To reach this goal, we performed specific cell culture retrotransposition assays in cells overexpressing or ablated for ADAR2. These experiments unveil a novel function of ADAR2 as suppressor of L1 retrotransposition. Furthermore, we showed that ADAR2 binds the basal L1 RNP complex.Overall, these data support the role of ADAR2 as regulator of L1 life cycle.
Keywords: ADAR2; LINE-1; RNA editing; double-stranded RNA (dsRNA); retrotransposons.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


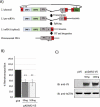



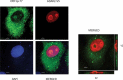
References
-
- Lander ES, Linton LM, Birren B, et al. International Human Genome Sequencing Consortium, Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
