Recurrent Frameshift Neoantigen Vaccine Elicits Protective Immunity With Reduced Tumor Burden and Improved Overall Survival in a Lynch Syndrome Mouse Model
- PMID: 34224739
- PMCID: PMC10184299
- DOI: 10.1053/j.gastro.2021.06.073
Recurrent Frameshift Neoantigen Vaccine Elicits Protective Immunity With Reduced Tumor Burden and Improved Overall Survival in a Lynch Syndrome Mouse Model
Erratum in
-
Correction.Gastroenterology. 2021 Dec;161(6):2070. doi: 10.1053/j.gastro.2021.10.008. Epub 2021 Oct 14. Gastroenterology. 2021. PMID: 34656532 No abstract available.
Abstract
Background & aims: DNA mismatch repair deficiency drives microsatellite instability (MSI). Cells with MSI accumulate numerous frameshift mutations. Frameshift mutations affecting cancer-related genes may promote tumorigenesis and, therefore, are shared among independently arising MSI tumors. Consequently, such recurrent frameshift mutations can give rise to shared immunogenic frameshift peptides (FSPs) that represent ideal candidates for a vaccine against MSI cancer. Pathogenic germline variants of mismatch repair genes cause Lynch syndrome (LS), a hereditary cancer syndrome affecting approximately 20-25 million individuals worldwide. Individuals with LS are at high risk of developing MSI cancer. Previously, we demonstrated safety and immunogenicity of an FSP-based vaccine in a phase I/IIa clinical trial in patients with a history of MSI colorectal cancer. However, the cancer-preventive effect of FSP vaccination in the scenario of LS has not yet been demonstrated.
Methods: A genome-wide database of 488,235 mouse coding mononucleotide repeats was established, from which a set of candidates was selected based on repeat length, gene expression, and mutation frequency. In silico prediction, in vivo immunogenicity testing, and epitope mapping was used to identify candidates for FSP vaccination.
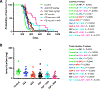
Results: We identified 4 shared FSP neoantigens (Nacad [FSP-1], Maz [FSP-1], Senp6 [FSP-1], Xirp1 [FSP-1]) that induced CD4/CD8 T cell responses in naïve C57BL/6 mice. Using VCMsh2 mice, which have a conditional knockout of Msh2 in the intestinal tract and develop intestinal cancer, we showed vaccination with a combination of only 4 FSPs significantly increased FSP-specific adaptive immunity, reduced intestinal tumor burden, and prolonged overall survival. Combination of FSP vaccination with daily naproxen treatment potentiated immune response, delayed tumor growth, and prolonged survival even more effectively than FSP vaccination alone.
Conclusions: Our preclinical findings support a clinical strategy of recurrent FSP neoantigen vaccination for LS cancer immunoprevention.
Keywords: Colorectal Cancer; Frameshift Neoantigens; Lynch Syndrome; Mouse Model; Preventive Cancer Vaccine.
Published by Elsevier Inc.
Conflict of interest statement
The rest of the authors do not report a conflict of interest.
Figures




References
-
- Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993;363:558–61. - PubMed
-
- Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260:816–9. - PubMed
-
- Kloor M, von Knebel Doeberitz, M. The immune biology of microsatellite-unstable cancer. Trends in Cancer 2016;2:121–131. - PubMed
-
- Markowitz S, Wang J, Myeroff L, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 1995;268:1336–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

