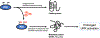Protein S-nitrosylation and oxidation contribute to protein misfolding in neurodegeneration
- PMID: 34224817
- PMCID: PMC8579830
- DOI: 10.1016/j.freeradbiomed.2021.07.002
Protein S-nitrosylation and oxidation contribute to protein misfolding in neurodegeneration
Abstract
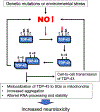
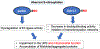
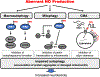
Neurodegenerative disorders like Alzheimer's disease and Parkinson's disease are characterized by progressive degeneration of synapses and neurons. Accumulation of misfolded/aggregated proteins represents a pathological hallmark of most neurodegenerative diseases, potentially contributing to synapse loss and neuronal damage. Emerging evidence suggests that misfolded proteins accumulate in the diseased brain at least in part as a consequence of excessively generated reactive oxygen species (ROS) and reactive nitrogen species (RNS). Mechanistically, not only disease-linked genetic mutations but also known risk factors for neurodegenerative diseases, such as aging and exposure to environmental toxins, can accelerate production of ROS/RNS, which contribute to protein misfolding - in many cases mimicking the effect of rare genetic mutations known to be linked to the disease. This review will focus on the role of RNS-dependent post-translational modifications, such as S-nitrosylation and tyrosine nitration, in protein misfolding and aggregation. Specifically, we will discuss molecular mechanisms whereby RNS disrupt the activity of the cellular protein quality control machinery, including molecular chaperones, autophagy/lysosomal pathways, and the ubiquitin-proteasome system (UPS). Because chronic accumulation of misfolded proteins can trigger mitochondrial dysfunction, synaptic damage, and neuronal demise, further characterization of RNS-mediated protein misfolding may establish these molecular events as therapeutic targets for intervention in neurodegenerative diseases.
Keywords: Autophagy; Molecular chaperones; Protein S-nitrosylation; Protein misfolding; Tyrosine nitration; Ubiquitin-proteasome system.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest
The authors declare that they have no conflicts of interest with the contents of this article.
Figures