Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials
- PMID: 34225791
- PMCID: PMC8256217
- DOI: 10.1186/s40249-021-00878-5
Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Various modalities of vaccines against coronavirus disease 2019 (COVID-19), based on different platforms and immunization procedures, have been successively approved for marketing worldwide. A comprehensive review for clinical trials assessing the safety of COVID-19 vaccines is urgently needed to make an accurate judgment for mass vaccination.
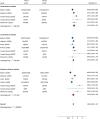
Main text: A systematic review and meta-analysis was conducted to determine the safety of COVID-19 vaccine candidates in randomized controlled trials (RCTs). Data search was performed in PubMed, Embase, Cochrane library, Scopus, Web of Science, and MedRxiv. Included articles were limited to RCTs on COVID-19 vaccines. A total of 73,633 subjects from 14 articles were included to compare the risks of adverse events following immunization (AEFI) after vaccinating different COVID-19 vaccines. Pooled risk ratios (RR) of total AEFI for inactivated vaccine, viral-vectored vaccine, and mRNA vaccine were 1.34 [95% confidence interval (CI) 1.11-1.61, P < 0.001], 1.65 (95% CI 1.31-2.07, P < 0.001), and 2.01 (95% CI 1.78-2.26, P < 0.001), respectively. No significant differences on local and systemic AEFI were found between the first dose and second dose. In addition, people aged ≤ 55 years were at significantly higher risk of AEFI than people aged ≥ 56 years, with a pooled RR of 1.25 (95% CI 1.15-1.35, P < 0.001).
Conclusions: The safety and tolerance of current COVID-19 vaccine candidates are acceptable for mass vaccination, with inactivated COVID-19 vaccines candidates having the lowest reported AEFI. Long-term surveillance of vaccine safety is required, especially among elderly people with underlying medical conditions.
Keywords: Adverse events following immunization; COVID-19 vaccine; Meta-analysis; Randomized controlled trial; Safety.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- WHO. Weekly epidemiological update—9 March 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update---.... Accessed 15 Mar 2021.
-
- WH0. Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand.... Accessed 4 Mar 2021.
-
- Merz T. Russia approves world’s first Covid vaccine, with Putin saying one of his daughters has had it. https://www.telegraph.co.uk/news/2020/08/11/russia-approves-worlds-first.... Accessed 4 Mar 2021.
Publication types
MeSH terms
Substances
Grants and funding
- JSGG20200225152008136/Science and Technology Planning Project of Shenzhen Municipality
- 82041043/National Natural Science Foundation of China
- 2020T130150ZX/China Postdoctoral Science Foundation
- 82022064/Innovative Research Group Project of the National Natural Science Foundation of China
- 81703278/National Outstanding Youth Science Fund Project of National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

