Shades of grey: host phenotype dependent effect of urbanization on the bacterial microbiome of a wild mammal
- PMID: 34225812
- PMCID: PMC8256534
- DOI: 10.1186/s42523-021-00105-4
Shades of grey: host phenotype dependent effect of urbanization on the bacterial microbiome of a wild mammal
Abstract
Background: Host-associated microbiota are integral to the ecology of their host and may help wildlife species cope with rapid environmental change. Urbanization is a globally replicated form of severe environmental change which we can leverage to better understand wildlife microbiomes. Does the colonization of separate cities result in parallel changes in the intestinal microbiome of wildlife, and if so, does within-city habitat heterogeneity matter? Using 16S rRNA gene amplicon sequencing, we quantified the effect of urbanization (across three cities) on the microbiome of eastern grey squirrels (Sciurus carolinensis). Grey squirrels are ubiquitous in rural and urban environments throughout their native range, across which they display an apparent coat colour polymorphism (agouti, black, intermediate).
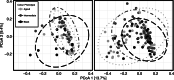
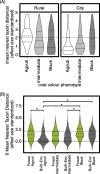
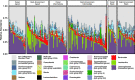
Results: Grey squirrel microbiomes differed between rural and city environments; however, comparable variation was explained by habitat heterogeneity within cities. Our analyses suggest that operational taxonomic unit (OTU) community structure was more strongly influenced by local environmental conditions (rural and city forests versus human built habitats) than urbanization of the broader landscape (city versus rural). The bacterial genera characterizing the microbiomes of built-environment squirrels are thought to specialize on host-derived products and have been linked in previous research to low fibre diets. However, despite an effect of urbanization at fine spatial scales, phylogenetic patterns in the microbiome were coat colour phenotype dependent. City and built-environment agouti squirrels displayed greater phylogenetic beta-dispersion than those in rural or forest environments, and null modelling results indicated that the phylogenetic structure of urban agouti squirrels did not differ greatly from stochastic expectations.
Conclusions: Squirrel microbiomes differed between city and rural environments, but differences of comparable magnitude were observed between land classes at a within-city scale. We did not observe strong evidence that inter-environmental differences were the result of disparate selective pressures. Rather, our results suggest that microbiota dispersal and ecological drift are integral to shaping the inter-environmental differences we observed. However, these processes were partly mediated by squirrel coat colour phenotype. Given a well-known urban cline in squirrel coat colour melanism, grey squirrels provide a useful free-living system with which to study how host genetics mediate environment x microbiome interactions.
Keywords: 16S rRNA gene; Colour polymorphism; Dispersal limitation; Eastern grey squirrel; Gene x environment interactions; Microbial ecology; Null modelling; Plasticity.
Conflict of interest statement
Not Applicable
Figures



References
LinkOut - more resources
Full Text Sources
