Paraventricular Nucleus Oxytocin Subsystems Promote Active Paternal Behaviors in Mandarin Voles
- PMID: 34226275
- PMCID: PMC8336703
- DOI: 10.1523/JNEUROSCI.2864-20.2021
Paraventricular Nucleus Oxytocin Subsystems Promote Active Paternal Behaviors in Mandarin Voles
Abstract
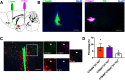
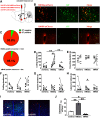
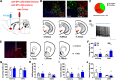
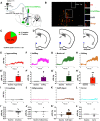
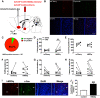
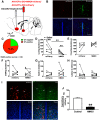
Paternal care plays a critical role in the development of brain and behaviors in offspring in monogamous species. However, the neurobiological mechanisms, especially the neuronal circuity, underlying paternal care is largely unknown. Using socially monogamous male mandarin voles (Microtus mandarinus) with high levels of paternal care, we found that paraventricular nucleus of the hypothalamus (PVN) to ventral tegmental area (VTA) or nucleus accumbens (NAc) oxytocin (OT) neurons are activated during paternal care. Chemogenetic activation/inhibition of the PVN OT projection to VTA promoted/decreased paternal care, respectively. Chemogenetic inhibition of the PVN to VTA OT pathway reduced dopamine (DA) release in the NAc of male mandarin voles during licking and grooming of pups as revealed by in vivo fiber photometry. Optogenetic activation/inhibition of the VTA to NAc DA pathway possibly enhanced/suppressed paternal behaviors, respectively. Furthermore, chemogenetic activation/inhibition of PVN to NAc OT circuit enhanced/inhibited paternal care. This finding is a first step toward delineating the neuronal circuity underlying paternal care and may have implications for treating abnormalities in paternal care associated with paternal postpartum depression or paternal abuse.SIGNIFICANCE STATEMENT Paternal behavior is essential for offspring survival and development in some mammalian species. However, the circuit mechanisms underlying the paternal brain are poorly understood. We show that manipulation of paraventricular nucleus of the hypothalamus (PVN) to ventral tegmental area (VTA) oxytocin (OT) projections as well as VTA to nucleus accumbens (NAc) DA projections promote paternal behaviors. Inhibition the PVN to VTA OT pathway reduces DA release in the NAc during pup licking and grooming. PVN to NAc OT circuit is also essential for paternal behaviors. Our findings identify two new neural circuits that modulate paternal behaviors.
Keywords: dopamine; nucleus accumbens; oxytocin; paraventricular nucleus of the hypothalamus; paternal behavior; ventral tegmental area.
Copyright © 2021 the authors.
Figures



References
-
- Bartz JA (2016) Oxytocin and the pharmacological dissection of affiliation. Curr Dir Psychol Sci 25:104–110. 10.1177/0963721415626678 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
