Timing of steroid initiation and response rates to immune checkpoint inhibitors in metastatic cancer
- PMID: 34226279
- PMCID: PMC8258666
- DOI: 10.1136/jitc-2020-002261
Timing of steroid initiation and response rates to immune checkpoint inhibitors in metastatic cancer
Abstract
Background: Corticosteroids (CS) are the mainstay of immune-related adverse effect (irAE) management, as well as for other indications in cancer treatment. Previous studies evaluating whether CS affect immune checkpoint inhibitor (CPI) efficacy compared patients receiving CS versus no CS. However, there is a paucity of clinical data evaluating the timing of concomitant CS and CPI efficacy.
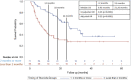
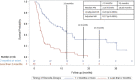
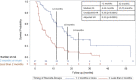
Methods: We retrospectively collected data from patients who received CS during CPI treatment at a single institution. Patients were in two cohorts based on timing of initiation of CS (≥2 months vs <2 months after initiating CPI). Patient characteristics, irAEs, cancer type, treatment type, treatment response/progression per RECIST V.1.1, and survival data were collected. Kaplan-Meier and Cox proportional hazard regression methods estimated HRs for the primary endpoint of progression-free survival (PFS) along with overall survival (OS).
Results: We identified 247 patients with metastatic cancer who received CS concurrently with CPIs. The median time on CS was 1.8 months. After adjusting for treatment type, tumor type, brain metastases, and irAEs, those treated with CS ≥2 months after starting CPI had a statistically significant longer PFS (HR=0.30, p<0.001), and OS (HR 0.34, p<0.0001) than those who received CS <2 months after starting CPI. Objective response rate (ORR) for patients on CS ≥2 months was 39.8%, versus ORR for patients <2 months was 14.7% (p value =<0.001) CONCLUSION: Our results suggest that early use of CS during CPI treatment significantly hinders CPI efficacy. This data needs to be validated prospectively. Future studies should focus on the immune mechanisms by which CSs affect T-cell function early in the CPI treatment course.
Keywords: immunotherapy; programmed cell death 1 receptor.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: There are no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
