Antioxidant Activity of Novel Casein-Derived Peptides with Microbial Proteases as Characterized via Keap1-Nrf2 Pathway in HepG2 Cells
- PMID: 34226415
- PMCID: PMC9705968
- DOI: 10.4014/jmb.2104.04013
Antioxidant Activity of Novel Casein-Derived Peptides with Microbial Proteases as Characterized via Keap1-Nrf2 Pathway in HepG2 Cells
Abstract
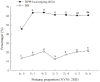
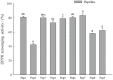
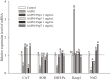
Casein-derived antioxidant peptides by using microbial proteases have gained increasing attention. Combination of two microbial proteases, Protin SD-NY10 and Protease A "Amano" 2SD, was employed to hydrolyze casein to obtain potential antioxidant peptides that were identified by LCMS/ MS, chemically synthesized and characterized in a oxidatively damaged HepG2 cell model. Four peptides, YQLD, FSDIPNPIGSEN, FSDIPNPIGSE, YFYP were found to possess high 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging ability. Evaluation with HepG2 cells showed that the 4 peptides at low concentrations (< 1.0 mg/ml) protected the cells against oxidative damage. The 4 peptides exhibited different levels of antioxidant activity by stimulating mRNA and protein expression of the antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px), as well as nuclear factor erythroid-2-related factor 2 (Nrf2), but decreasing the mRNA expression of Kelch-like ECH-associated protein 1 (Keap1). Furthermore, these peptides decreased production of reactive oxygen species (ROS) and malondialdehyde (MDA), but increased glutathione (GSH) production in HepG2 cells. Therefore, the 4 casein-derived peptides obtained by using microbial proteases exhibited different antioxidant activity by activating the Keap1-Nrf2 signaling pathway, and they could serve as potential antioxidant agents in functional foods or pharmaceutic preparation.
Keywords: HepG2 cells; Keap1-Nrf2 pathway; Microbial protease; antioxidant peptide; casein hydrolysate.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures





References
-
- Silva SV, Malcata FX. Caseins as source of bioactive peptides. Int. Dairy J. 2005;15:1–15. doi: 10.1016/j.idairyj.2004.04.009. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

