Breast cancer brain metastasis: insight into molecular mechanisms and therapeutic strategies
- PMID: 34226684
- PMCID: PMC8505648
- DOI: 10.1038/s41416-021-01424-8
Breast cancer brain metastasis: insight into molecular mechanisms and therapeutic strategies
Abstract
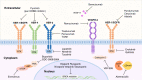
Breast cancer is one of the most prevalent malignancies in women worldwide. Early-stage breast cancer is considered a curable disease; however, once distant metastasis occurs, the 5-year overall survival rate of patients becomes significantly reduced. There are four distinct metastatic patterns in breast cancer: bone, lung, liver and brain. Among these, breast cancer brain metastasis (BCBM) is the leading cause of death; it is highly associated with impaired quality of life and poor prognosis due to the limited permeability of the blood-brain barrier and consequent lack of effective treatments. Although the sequence of events in BCBM is universally accepted, the underlying mechanisms have not yet been fully elucidated. In this review, we outline progress surrounding the molecular mechanisms involved in BCBM as well as experimental methods and research models to better understand the process. We further discuss the challenges in the management of brain metastases, as well as providing an overview of current therapies and highlighting innovative research towards developing novel efficacious targeted therapies.
© 2021. The Author(s), under exclusive licence to Cancer Research UK.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

