Lyme arthritis: linking infection, inflammation and autoimmunity
- PMID: 34226730
- PMCID: PMC9488587
- DOI: 10.1038/s41584-021-00648-5
Lyme arthritis: linking infection, inflammation and autoimmunity
Abstract
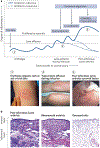
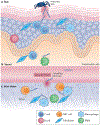
Infectious agents can trigger autoimmune responses in a number of chronic inflammatory diseases. Lyme arthritis, which is caused by the tick-transmitted spirochaete Borrelia burgdorferi, is effectively treated in most patients with antibiotic therapy; however, in a subset of patients, arthritis can persist and worsen after the spirochaete has been killed (known as post-infectious Lyme arthritis). This Review details the current understanding of the pathogenetic events in Lyme arthritis, from initial infection in the skin, through infection of the joints, to post-infectious chronic inflammatory arthritis. The central feature of post-infectious Lyme arthritis is an excessive, dysregulated pro-inflammatory immune response during the infection phase that persists into the post-infectious period. This response is characterized by high amounts of IFNγ and inadequate amounts of the anti-inflammatory cytokine IL-10. The consequences of this dysregulated pro-inflammatory response in the synovium include impaired tissue repair, vascular damage, autoimmune and cytotoxic processes, and fibroblast proliferation and fibrosis. These synovial characteristics are similar to those in other chronic inflammatory arthritides, including rheumatoid arthritis. Thus, post-infectious Lyme arthritis provides a model for other chronic autoimmune or autoinflammatory arthritides in which complex immune responses can be triggered and shaped by an infectious agent in concert with host genetic factors.
© 2021. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures




References
-
- von Herrath MG, Fujinami RS & Whitton JL Microorganisms and autoimmunity: making the barren field fertile? Nat. Rev. Microbiol 1, 151–157 (2003). - PubMed
-
- Steere AC Lyme disease. N. Engl. J. Med 321, 586–596 (1989). - PubMed
-
- Steere AC, Schoen RT & Taylor E The clinical evolution of Lyme arthritis. Ann. Intern. Med 107, 725–731 (1987). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

