Protein-lipid interplay at the neuromuscular junction
- PMID: 34226930
- PMCID: PMC8855523
- DOI: 10.1093/jmicro/dfab023
Protein-lipid interplay at the neuromuscular junction
Abstract
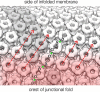
Many new structures of membrane proteins have been determined over the last decade, yet the nature of protein-lipid interplay has received scant attention. The postsynaptic membrane of the neuromuscular junction and Torpedo electrocytes has a regular architecture, opening an opportunity to illuminate how proteins and lipids act together in a native membrane setting. Cryo electron microscopy (Cryo-EM) images show that cholesterol segregates preferentially around the constituent ion channel, the nicotinic acetylcholine receptor, interacting with specific sites in both leaflets of the bilayer. In addition to maintaining the transmembrane α-helical architecture, cholesterol forms microdomains - bridges of rigid sterol groups that link one channel to the next. This article discusses the whole protein-lipid organization of the cholinergic postsynaptic membrane, its physiological implications and how the observed details relate to our current concept of the membrane structure. I suggest that cooperative interactions, facilitated by the regular protein-lipid arrangement, help to spread channel activation into regions distant from the sites of neurotransmitter release, thereby enhancing the postsynaptic response.
Keywords: cholesterol microdomain; cryo-EM; nicotinic acetylcholine receptor; postsynaptic membrane; synaptic transmission.
© The Author(s) 2022. Published by Oxford University Press on behalf of The Japanese Society of Microscopy.
Figures





References
-
- Baenziger J E, Domville J A, and Therien J P D (2017) The role of cholesterol in the activation of nicotinic acetylcholine receptors. Curr. Top. Membr. 80: 95–137. - PubMed
-
- Barrantes F J (2010) Cholesterol effects on nicotinic acetylcholine receptor: cellular aspects. Subcell. Biochem. 51: 467–487. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

