One-Year Landmark Analysis of the Effect of Beta-Blocker Dose on Survival After Acute Myocardial Infarction
- PMID: 34227397
- PMCID: PMC8483468
- DOI: 10.1161/JAHA.120.019017
One-Year Landmark Analysis of the Effect of Beta-Blocker Dose on Survival After Acute Myocardial Infarction
Abstract
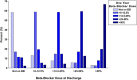
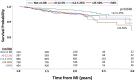
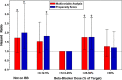
Background Although beta-blockers are recommended following myocardial infarction (MI), the benefits of long-term treatment have not been established. The study's aim was to evaluate beta-blocker efficacy by dose in 1-year post-MI survivors. Methods and Results The OBTAIN (Outcomes of Beta-Blocker Therapy After Myocardial Infarction) registry included 7057 patients with acute MI, with 6077 one-year survivors. For this landmark analysis, beta-blocker dose status was available in 3004 patients and analyzed by use (binary) and dose at 1 year after MI. Doses were classified as no beta-blocker and >0% to 12.5%, >12.5% to 25%, >25% to 50%, and >50% of target doses used in randomized clinical trials. Age was 63 to 64 years, and approximately two thirds were men. Median follow-up duration was 1.05 years (interquartile range, 0.98-1.22). When analyzed dichotomously, beta-blocker therapy was not associated with improved survival. When analyzed by dose, propensity score analysis showed significantly increased mortality in the no-beta-blocker group (hazard ratio,1.997; 95% CI, 1.118-3.568; P<0.02), the >0% to 12.5% group (hazard ratio, 1.817; 95% CI, 1.094-3.016; P<0.02), and the >25% to 50% group (hazard ratio, 1.764; 95% CI, 1.105-2.815; P<0.02), compared with the >12.5% to 25% dose group. The mortality in the full-dose group was not significantly higher (hazard ratio, 1.196; 95% CI, 0.687-2.083). In subgroup analyses, only history of congestive heart failure demonstrated significant interaction with beta-blocker effects on survival. Conclusions This analysis suggests that patients treated with >12.5% to 25% of the target dose used in prior randomized clinical trials beyond 1 year after MI may have enhanced survival compared with no beta-blocker and other beta-blocker doses. A new paradigm for post-MI beta-blocker therapy is needed that addresses which patients should be treated, for how long, and at what dose.
Keywords: beta‐blocker; landmark analysis; myocardial infarction; survival.
Conflict of interest statement
None.
Figures
Comment in
-
Pharmacological Treatment Following Myocardial Infarction: How Large Is the Gap Between Guideline Recommendations and Routine Clinical Care?J Am Heart Assoc. 2021 Jul 20;10(14):e021799. doi: 10.1161/JAHA.121.021799. Epub 2021 Jul 6. J Am Heart Assoc. 2021. PMID: 34227398 Free PMC article. No abstract available.
References
-
- Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e344–e426. DOI: 10.1161/CIR.0000000000000134. - DOI - PubMed
-
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–555. DOI: 10.1161/CIR.0b013e3182742c84. - DOI - PubMed
-
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. DOI: 10.1093/eurheartj/ehx393. - DOI - PubMed
-
- Roffi M, Patrono C, Collet J‐P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. DOI: 10.1093/eurheartj/ehv320. - DOI - PubMed
-
- Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. Circulation. 2011;124:2458–2473. DOI: 10.1161/CIR.0b013e318235eb4d. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

