Biosensing with DNAzymes
- PMID: 34227631
- PMCID: PMC9136875
- DOI: 10.1039/d1cs00240f
Biosensing with DNAzymes
Abstract
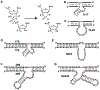
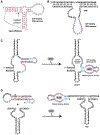
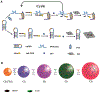
This article provides a comprehensive review of biosensing with DNAzymes, providing an overview of different sensing applications while highlighting major progress and seminal contributions to the field of portable biosensor devices and point-of-care diagnostics. Specifically, the field of functional nucleic acids is introduced, with a specific focus on DNAzymes. The incorporation of DNAzymes into bioassays is then described, followed by a detailed overview of recent advances in the development of in vivo sensing platforms and portable sensors incorporating DNAzymes for molecular recognition. Finally, a critical perspective on the field, and a summary of where DNAzyme-based devices may make the biggest impact are provided.
Conflict of interest statement
Conflicts of interest
Yi Lu is a co-founder of ANDalyze Inc. and GlucoSentient Inc.; Yingfu Li is a co-founder of Innovogene Biosciences Inc.
Figures
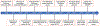











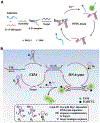

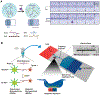





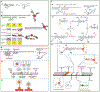
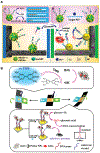




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

