Clinical Evaluation of a Novel Wearable Compression Technology in the Treatment of Lymphedema, an Open-Label Controlled Study
- PMID: 34227842
- PMCID: PMC9081034
- DOI: 10.1089/lrb.2020.0126
Clinical Evaluation of a Novel Wearable Compression Technology in the Treatment of Lymphedema, an Open-Label Controlled Study
Abstract
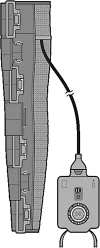
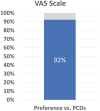
A diagnosis of lymphedema comes with a lifetime requirement for careful self-care and treatment to control skin deterioration and the consequences of excessive fluid and protein buildup leading to abnormal limb volume and an increased risk of infection. The burden of care and psychosocial aspects of physical disfiguration and loss of function are associated with compromised quality of life (QoL). The current standard therapeutic intervention is complex decongestive therapy with manual lymph drainage and frequent wearing of compression garments. With insurance limitations on therapy visits and the time and travel required, additional home treatment options are needed. Pneumatic compression pumps that mimic the manual massage pressure and pattern are sometimes prescribed, but these are bulky, difficult to apply, and require immobility during treatment. An open-label pilot study in 40 subjects was performed to evaluate the QoL and limb volume maintenance efficacy of a novel wearable compression system (Dayspring™) that is low profile, easy to use, and allows for mobility during treatment. After 28 days of use, subjects had a statistically significant 18% (p < 0.001) improvement in overall QoL as measured by the Lymphedema Quality-of-Life Questionnaire compared with baseline. Individual QoL domains, and limb volume improved with therapy. Adherence was 98% over the course of the study. Results of the clinical evaluation suggest the Dayspring wearable compression device is safe and effective and improves QoL and limb volume. The novel, low-profile device is easy to use and allows for mobility during treatment, addressing a potential barrier to adherence with pneumatic compression devices.
Keywords: advanced compression; lymphedema; mobility; quality of life; wearable.
Conflict of interest statement
Dr. S.G.R., Dr. P.K.-M., Dr. R.S., and Dr. J.A. serve as advisors to Koya Medical. Over the past 24 months, Dr. P.K.-M. reports providing consulting services to Precision Health Economics and Sempre Health for work unrelated to this study. The authors have no financial interests in the study.
Figures



References
-
- Underlying incidence data are from the SEER 9 areas. http://seer.cancer.gov/registries/terms.html (accessed June 29, 2021).
-
- Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: A site-specific analysis of the SEER database. Int J Cancer 2005; 114:806–816. - PubMed
-
- Hayes SC. Review of Research Evidence on Secondary Lymphoedema—Incidence, Prevention, Risk Factors and Treatment. NSW: National Breast and Ovarian Cancer Centre Surry Hills; 2008:1–83.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
