Association of Apolipoprotein E ɛ4 Allele With Clinical and Multimodal Biomarker Changes of Alzheimer Disease in Adults With Down Syndrome
- PMID: 34228042
- PMCID: PMC8261691
- DOI: 10.1001/jamaneurol.2021.1893
Association of Apolipoprotein E ɛ4 Allele With Clinical and Multimodal Biomarker Changes of Alzheimer Disease in Adults With Down Syndrome
Abstract
Importance: Alzheimer disease (AD) is the leading cause of death in individuals with Down syndrome (DS). Previous studies have suggested that the APOE ɛ4 allele plays a role in the risk and age at onset of dementia in DS; however, data on in vivo biomarkers remain scarce.
Objective: To investigate the association of the APOE ɛ4 allele with clinical and multimodal biomarkers of AD in adults with DS.
Design, setting, and participants: This dual-center cohort study recruited adults with DS in Barcelona, Spain, and in Cambridge, UK, between June 1, 2009, and February 28, 2020. Included individuals had been genotyped for APOE and had at least 1 clinical or AD biomarker measurement; 2 individuals were excluded because of the absence of trisomy 21. Participants were either APOE ɛ4 allele carriers or noncarriers.
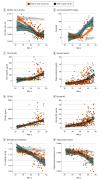
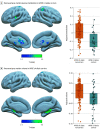
Main outcomes and measures: Participants underwent a neurological and neuropsychological assessment. A subset of participants had biomarker measurements: Aβ1-42, Aβ1-40, phosphorylated tau 181 (pTau181) and neurofilament light chain (NfL) in cerebrospinal fluid (CSF), pTau181, and NfL in plasma; amyloid positron emission tomography (PET); fluorine 18-labeled-fluorodeoxyglucose PET; and/or magnetic resonance imaging. Age at symptom onset was compared between APOE ɛ4 allele carriers and noncarriers, and within-group local regression models were used to compare the association of biomarkers with age. Voxelwise analyses were performed to assess topographical differences in gray matter metabolism and volume.
Results: Of the 464 adults with DS included in the study, 97 (20.9%) were APOE ɛ4 allele carriers and 367 (79.1%) were noncarriers. No differences between the 2 groups were found by age (median [interquartile range], 45.9 [36.4-50.2] years vs 43.7 [34.9-50.2] years; P = .56) or sex (51 male carriers [52.6%] vs 199 male noncarriers [54.2%]). APOE ɛ4 allele carriers compared with noncarriers presented with AD symptoms at a younger age (mean [SD] age, 50.7 [4.4] years vs 52.7 [5.8] years; P = .02) and showed earlier cognitive decline. Locally estimated scatterplot smoothing curves further showed between-group differences in biomarker trajectories with age as reflected by nonoverlapping CIs. Specifically, carriers showed lower levels of the CSF Aβ1-42 to Aβ1-40 ratio until age 40 years, earlier increases in amyloid PET and plasma pTau181, and earlier loss of cortical metabolism and hippocampal volume. No differences were found in NfL biomarkers or CSF total tau and pTau181. Voxelwise analyses showed lower metabolism in subcortical and parieto-occipital structures and lower medial temporal volume in APOE ɛ4 allele carriers.
Conclusions and relevance: In this study, the APOE ɛ4 allele was associated with earlier clinical and biomarker changes of AD in DS. These results provide insights into the mechanisms by which APOE increases the risk of AD, emphasizing the importance of APOE genotype for future clinical trials in DS.
Conflict of interest statement
Figures

Comment in
-
APOE ε4 Association With Cognition and Alzheimer Disease Biomarkers in Down Syndrome-Implications for Clinical Trials and Treatments for All.JAMA Neurol. 2021 Aug 1;78(8):913-915. doi: 10.1001/jamaneurol.2021.1649. JAMA Neurol. 2021. PMID: 34228117 No abstract available.

