Functional Outcomes Over the First Year After Moderate to Severe Traumatic Brain Injury in the Prospective, Longitudinal TRACK-TBI Study
- PMID: 34228047
- PMCID: PMC8261688
- DOI: 10.1001/jamaneurol.2021.2043
Functional Outcomes Over the First Year After Moderate to Severe Traumatic Brain Injury in the Prospective, Longitudinal TRACK-TBI Study
Abstract
Importance: Moderate to severe traumatic brain injury (msTBI) is a major cause of death and disability in the US and worldwide. Few studies have enabled prospective, longitudinal outcome data collection from the acute to chronic phases of recovery after msTBI.
Objective: To prospectively assess outcomes in major areas of life function at 2 weeks and 3, 6, and 12 months after msTBI.
Design, setting, and participants: This cohort study, as part of the Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) study, was conducted at 18 level 1 trauma centers in the US from February 2014 to August 2018 and prospectively assessed longitudinal outcomes, with follow-up to 12 months postinjury. Participants were patients with msTBI (Glasgow Coma Scale scores 3-12) extracted from a larger group of patients with mild, moderate, or severe TBI who were enrolled in TRACK-TBI. Data analysis took place from October 2019 to April 2021.
Exposures: Moderate or severe TBI.
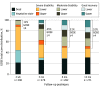
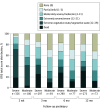
Main outcomes and measures: The Glasgow Outcome Scale-Extended (GOSE) and Disability Rating Scale (DRS) were used to assess global functional status 2 weeks and 3, 6, and 12 months postinjury. Scores on the GOSE were dichotomized to determine favorable (scores 4-8) vs unfavorable (scores 1-3) outcomes. Neurocognitive testing and patient reported outcomes at 12 months postinjury were analyzed.
Results: A total of 484 eligible patients were included from the 2679 individuals in the TRACK-TBI study. Participants with severe TBI (n = 362; 283 men [78.2%]; median [interquartile range] age, 35.5 [25-53] years) and moderate TBI (n = 122; 98 men [80.3%]; median [interquartile range] age, 38 [25-53] years) were comparable on demographic and premorbid variables. At 2 weeks postinjury, 36 of 290 participants with severe TBI (12.4%) and 38 of 93 participants with moderate TBI (41%) had favorable outcomes (GOSE scores 4-8); 301 of 322 in the severe TBI group (93.5%) and 81 of 103 in the moderate TBI group (78.6%) had moderate disability or worse on the DRS (total score ≥4). By 12 months postinjury, 142 of 271 with severe TBI (52.4%) and 54 of 72 with moderate TBI (75%) achieved favorable outcomes. Nearly 1 in 5 participants with severe TBI (52 of 270 [19.3%]) and 1 in 3 with moderate TBI (23 of 71 [32%]) reported no disability (DRS score 0) at 12 months. Among participants in a vegetative state at 2 weeks, 62 of 79 (78%) regained consciousness and 14 of 56 with available data (25%) regained orientation by 12 months.
Conclusions and relevance: In this study, patients with msTBI frequently demonstrated major functional gains, including recovery of independence, between 2 weeks and 12 months postinjury. Severe impairment in the short term did not portend poor outcomes in a substantial minority of patients with msTBI. When discussing prognosis during the first 2 weeks after injury, clinicians should be particularly cautious about making early, definitive prognostic statements suggesting poor outcomes and withdrawal of life-sustaining treatment in patients with msTBI.
Conflict of interest statement
Figures

References
-
- Centers for Disease Control and Prevention, US Department of Health and Human Services . Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2014. Published 2019. Accessed June 3, 2021. https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-FIN...
-
- Kumar RG, Ketchum JM, Corrigan JD, Hammond FM, Sevigny M, Dams-O’Connor K. The longitudinal effects of comorbid health burden on functional outcomes for adults with moderate to severe traumatic brain injury. J Head Trauma Rehabil. 2020;35(4):E372-E381. doi: 10.1097/HTR.0000000000000572 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

