Effect of Cytisine vs Varenicline on Smoking Cessation: A Randomized Clinical Trial
- PMID: 34228066
- PMCID: PMC8261608
- DOI: 10.1001/jama.2021.7621
Effect of Cytisine vs Varenicline on Smoking Cessation: A Randomized Clinical Trial
Abstract
Importance: Cytisine is more effective than placebo and nicotine replacement therapy for smoking cessation. However, cytisine has not been tested against the most effective smoking cessation medication, varenicline, which is associated with adverse events known to lead to discontinuation of therapy.
Objective: To examine whether standard cytisine treatment (25 days) was at least as effective as standard varenicline treatment (84 days) for smoking cessation.
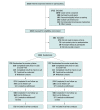
Design, setting, and participants: This noninferiority, open-label randomized clinical trial with allocation concealment and blinded outcome assessment was undertaken in Australia from November 2017 through May 2019; follow-up was completed in January 2020. A total of 1452 Australian adult daily smokers willing to make a quit attempt were included. Data collection was conducted primarily by computer-assisted telephone interview, but there was an in-person visit to validate the primary outcome.
Interventions: Treatments were provided in accordance with the manufacturers' recommended dosage: cytisine (n = 725), 1.5-mg capsules taken 6 times daily initially then gradually reduced over the 25-day course; varenicline (n = 727), 0.5-mg tablets titrated to 1 mg twice daily for 84 days (12 weeks). All participants were offered referral to standard telephone behavioral support.
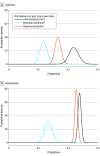
Main outcomes and measures: The primary outcome was 6-month continuous abstinence verified using a carbon monoxide breath test at 7-month follow-up. The noninferiority margin was set at 5% and the 1-sided significance threshold was set at .025.
Results: Among 1452 participants who were randomized (mean [SD] age, 42.9 [12.7] years; 742 [51.1%] women), 1108 (76.3%) completed the trial. Verified 6-month continuous abstinence rates were 11.7% for the cytisine group and 13.3% for the varenicline group (risk difference, -1.62% [1-sided 97.5% CI, -5.02% to ∞]; P = .03 for noninferiority). Self-reported adverse events occurred less frequently in the cytisine group (997 events among 482 participants) compared with the varenicline group (1206 events among 510 participants) and the incident rate ratio was 0.88 (95% CI, 0.81 to 0.95; P = .002).
Conclusions and relevance: Among daily smokers willing to quit, cytisine treatment for 25 days, compared with varenicline treatment for 84 days, failed to demonstrate noninferiority regarding smoking cessation.
Trial registration: anzctr.org.au Identifier: ACTRN12616001654448.
Conflict of interest statement
Figures
Comment in
-
The Effect of Cytisine vs Varenicline on Smoking Cessation.JAMA. 2021 Nov 9;326(18):1872. doi: 10.1001/jama.2021.16046. JAMA. 2021. PMID: 34751716 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

