Effect of Antibiotic-Prescribing Feedback to High-Volume Primary Care Physicians on Number of Antibiotic Prescriptions: A Randomized Clinical Trial
- PMID: 34228086
- PMCID: PMC8261687
- DOI: 10.1001/jamainternmed.2021.2790
Effect of Antibiotic-Prescribing Feedback to High-Volume Primary Care Physicians on Number of Antibiotic Prescriptions: A Randomized Clinical Trial
Abstract
Importance: Antibiotic overuse contributes to adverse drug effects, increased costs, and antimicrobial resistance.
Objective: To evaluate peer-comparison audit and feedback to high-prescribing primary care physicians (PCPs) and assess the effect of targeted messaging on avoiding unnecessary antibiotic prescriptions and avoiding long-duration prescribing.
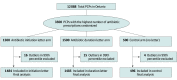
Design, setting, and participants: In this 3-arm randomized clinical trial, administrative data collected from IQVIA's Xponent database were used to recruit the highest quartile of antibiotic-prescribing PCPs (n = 3500) in Ontario, Canada.
Interventions: Physicians were randomized 3:3:1 to receive a mailed letter sent in December 2018 addressing antibiotic treatment initiation (n = 1500), a letter addressing prescribing duration (n = 1500), or no letter (control; n = 500). Outliers at the 99th percentile at baseline for each arm were excluded from analysis.
Main outcomes and measures: The primary outcome was total number of antibiotic prescriptions over 12 months postintervention. Secondary outcomes were number of prolonged-duration prescriptions (>7 days) and antibiotic drug costs (in Canadian dollars). Robust Poisson regression controlling for baseline prescriptions was used for analysis.
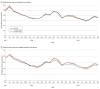
Results: Of the 3465 PCPs included in analysis, 2405 (69.4%) were male, and 2116 (61.1%) were 25 or more years from their medical graduation. At baseline, PCPs receiving the antibiotic initiation letter and duration letter prescribed an average of 988 and 1000 antibiotic prescriptions, respectively; at 12 months postintervention, these PCPs prescribed an average of 849 and 851 antibiotic prescriptions, respectively. For the primary outcome of total antibiotic prescriptions 12 months postintervention, there was no statistically significant difference in total antibiotic use between PCPs who received the initiation letter compared with controls (relative risk [RR], 0.96; 97.5% CI, 0.92-1.01; P = .06) and a small statistically significant difference for the duration letter compared with controls (RR, 0.95; 97.5% CI, 0.91-1.00; P = .01). For PCPs receiving the duration letter, this corresponds to an average of 42 fewer antibiotic prescriptions over 12 months. There was no statistically significant difference between the letter arms. For the initiation letter, compared with controls there was an RR of 0.98 (97.5% CI, 0.93-1.03; P = .42) and 0.97 (97.5% CI, 0.92-1.02; P = .19) for the outcomes of prolonged-duration prescriptions and antibiotic drug costs, respectively. At baseline, PCPs who received the duration letter prescribed an average of 332 prolonged-duration prescriptions with $14 470 in drug costs. There was an 8.1% relative reduction (RR, 0.92; 97.5% CI, 0.87-0.97; P < .001) in prolonged-duration prescriptions, and a 6.1% relative reduction in antibiotic drug costs (RR, 0.94; 97.5% CI, 0.89-0.99; P = .01). This corresponds to an average of 24 fewer prolonged-duration prescriptions and $771 in drug cost savings per PCP over 12 months.
Conclusions and relevance: In this randomized clinical trial, a single mailed letter to the highest-prescribing PCPs in Ontario, Canada providing peer-comparison feedback, including messaging on limiting antibiotic-prescribing durations, led to statistically significant reductions in total and prolonged-duration antibiotic prescriptions, as well as drug costs.
Trial registration: ClinicalTrials.gov Identifier: NCT03776383.
Conflict of interest statement
Figures

Comment in
-
Socioeconomic Factors, Urological Epidemiology and Practice Patterns.J Urol. 2022 Mar;207(3):724-725. doi: 10.1097/JU.0000000000002373. Epub 2021 Dec 16. J Urol. 2022. PMID: 34911340 No abstract available.
-
Socioeconomic Factors, Urological Epidemiology, and Practice Patterns.J Urol. 2023 Aug;210(2):371-373. doi: 10.1097/JU.0000000000003534. Epub 2023 May 16. J Urol. 2023. PMID: 37192388 No abstract available.
References
-
- O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. Review on Antimicrobial Resistance. May 2016. Accessed May 24, 2021. https://www.biomerieuxconnection.com/wp-content/uploads/2018/04/Tackling...

