Detection of pre-existing SARS-CoV-2-reactive T cells in unexposed renal transplant patients
- PMID: 34228322
- PMCID: PMC8259083
- DOI: 10.1007/s40620-021-01092-0
Detection of pre-existing SARS-CoV-2-reactive T cells in unexposed renal transplant patients
Abstract
Background: Recent data demonstrate potentially protective pre-existing T cells reactive against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in samples of healthy blood donors, collected before the SARS-CoV-2 pandemic. Whether pre-existing immunity is also detectable in immunosuppressed patients is currently not known.
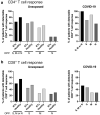
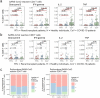
Methods: Fifty-seven patients were included in this case-control study. We compared the frequency of SARS-CoV-2-reactive T cells in the samples of 20 renal transplant (RTx) patients to 20 age/gender matched non-immunosuppressed/immune competent healthy individuals collected before the onset of the SARS-CoV-2 pandemic. Seventeen coronavirus disease 2019 (COVID-19) patients were used as positive controls. T cell reactivity against Spike-, Nucleocapsid-, and Membrane- SARS-CoV-2 proteins were analyzed by multi-parameter flow cytometry. Antibodies were analyzed by neutralization assay.
Results: Pre-existing SARS-CoV-2-reactive T cells were detected in the majority of unexposed patients and healthy individuals. In RTx patients, 13/20 showed CD4+ T cells reactive against at least one SARS-CoV-2 protein. CD8+ T cells reactive against at least one SARS-CoV-2 protein were demonstrated in 12/20 of RTx patients. The frequency and Th1 cytokine expression pattern of pre-formed SARS-CoV-2 reactive T cells did not differ between RTx and non-immunosuppressed healthy individuals.
Conclusions: This study shows that the magnitude and functionality of pre-existing SARS-CoV-2 reactive T cell in transplant patients is non-inferior compared to the immune competent cohort. Although several pro-inflammatory cytokines were produced by the detected T cells, further studies are required to prove their antiviral protection.
Keywords: Antigen-spcific T cells; Immunosuppression; Renal transplantation; Sars-CoV-2.
© 2021. The Author(s).
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose.
Figures



References
-
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. - DOI - PMC - PubMed
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- Banerjee A, Pasea L, Harris S, Gonzalez-Izquierdo A, Torralbo A, Shallcross L, Noursadeghi M, Pillay D, Sebire N, Holmes C, Pagel C, Wong WK, Langenberg C, Williams B, Denaxas S, Hemingway H. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet. 2020;395(10238):1715–1725. doi: 10.1016/S0140-6736(20)30854-0. - DOI - PMC - PubMed
-
- Thieme CJ, Anft M, Paniskaki K, Blazquez-Navarro A, Doevelaar A, Seibert FS, Hoelzer B, Konik MJ, Berger MM, Brenner T, Tempfer C, Watzl C, Meister TL, Pfaender S, Steinmann E, Dolff S, Dittmer U, Westhoff TH, Witzke O, Stervbo U, Roch T, Babel N. Robust T cell response toward spike, membrane, and nucleocapsid SARS-CoV-2 proteins is not associated with recovery in critical COVID-19 patients. Cell Rep Med. 2020;1(6):100092. doi: 10.1016/j.xcrm.2020.100092. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

