Trypanosoma cruzi Exploits E- and P-Selectins To Migrate Across Endothelial Cells and Extracellular Matrix Proteins
- PMID: 34228487
- PMCID: PMC8445167
- DOI: 10.1128/IAI.00178-21
Trypanosoma cruzi Exploits E- and P-Selectins To Migrate Across Endothelial Cells and Extracellular Matrix Proteins
Abstract
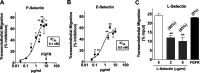
The Chagas disease parasite Trypanosoma cruzi must extravasate to home in on susceptible cells residing in most tissues. It remains unknown how T. cruzi undertakes this crucial step of its life cycle. We hypothesized that the pathogen exploits the endothelial cell programming leukocytes use to extravasate to sites of inflammation. Transendothelial migration (TEM) starts after inflammatory cytokines induce E-selectin expression and P-selectin translocation on endothelial cells (ECs), enabling recognition by leukocyte ligands that engender rolling cell adhesion. Here, we show that T. cruzi upregulates E- and P-selectins in cardiac ECs to which it binds in a ligand-receptor fashion, whether under static or shear flow conditions. Glycoproteins isolated from T. cruzi (TcEx) specifically recognize P-selectin in a ligand-receptor interaction. As with leukocytes, binding of P-selectin to T. cruzi or TcEx requires sialic acid and tyrosine sulfate, which are pivotal for downstream migration across ECs and extracellular matrix proteins. Additionally, soluble selectins, which bind T. cruzi, block transendothelial migration dose dependently, implying that the pathogen bears selectin-binding ligand(s) that start transmigration. Furthermore, function-blocking antibodies against E- and P-selectins, which act on endothelial cells and not T. cruzi, are exquisite in preventing TEM. Thus, our results show that selectins can function as mediators of T. cruzi transendothelial transmigration, suggesting a pathogenic mechanism that allows homing in of the parasite on targeted tissues. As selectin inhibitors are sought-after therapeutic targets for autoimmune diseases and cancer metastasis, they may similarly represent a novel strategy for Chagas disease therapy.
Keywords: Chagas disease; E-selectin; L-selectin; P-selectin; Trypanosoma cruzi; sialic acid; sulfate; transendothelial migration.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

