Identifying the Basis for VirS/VirR Two-Component Regulatory System Control of Clostridium perfringens Beta-Toxin Production
- PMID: 34228498
- PMCID: PMC8378477
- DOI: 10.1128/JB.00279-21
Identifying the Basis for VirS/VirR Two-Component Regulatory System Control of Clostridium perfringens Beta-Toxin Production
Abstract
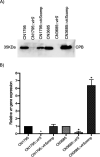
Clostridium perfringens toxin production is often regulated by the Agr-like quorum sensing (QS) system signaling the VirS/VirR two-component regulatory system (TCRS), which consists of the VirS membrane sensor histidine kinase and the VirR response regulator. VirS/VirR is known to directly control expression of some genes by binding to a DNA binding motif consisting of two VirR boxes located within 500 bp of the target gene start codon. Alternatively, the VirS/VirR system can indirectly regulate production levels of other proteins by increasing expression of a small regulatory RNA, VR-RNA. Previous studies demonstrated that C. perfringens beta-toxin (CPB) production by C. perfringens type B and C strains is positively regulated by both the Agr-like QS and the VirS/VirR TCRS, but the mechanism has been unclear. The current study first inactivated the vrr gene encoding VR-RNA to show that VirS/VirR regulation of cpb expression does not involve VR-RNA. Subsequently, bioinformatic analyses identified a potential VirR binding motif, along with a predicted strong promoter, ∼1.4 kb upstream of the cpb open reading frame (ORF). Two insertion sequences were present between this VirR binding motif/promoter region and the cpb ORF. PCR screening of a collection of strains carrying cpb showed that the presence and sequence of this VirR binding motif/promoter is highly conserved among CPB-producing strains. Reverse transcription-PCR (RT-PCR) and a GusA reporter assay showed this VirR binding motif is important for regulating CPB production. These findings indicate that VirS/VirR directly regulates cpb expression via VirS binding to a VirR binding motif located unusually distant from the cpb start codon. IMPORTANCE Clostridium perfringens beta-toxin (CPB) is only produced by type B and C strains. Production of CPB is essential for the pathogenesis of type C-associated infections, which include hemorrhagic necrotizing enteritis and enterotoxemia in both humans and animals. In addition, CPB can synergize with other toxins during C. perfringens gastrointestinal diseases. CPB toxin production is cooperatively regulated by the Agr-like quorum sensing (QS) system and the VirS/VirR two-component regulatory system. This study now reports that the VirS/VirR regulatory cascade directly controls expression of the cpb gene via a process involving a VirR box binding motif located unusually far (∼1.4 kb) upstream of the cpb ORF. This study provides a better understanding of the regulatory mechanisms for CPB production by the VirS/VirR regulatory cascade.
Keywords: Clostridium perfringens; VirR box; VirS/VirR; beta-toxin; gene regulation; insertion sequences; regulatory system; two-component regulatory system.
Figures

Similar articles
-
Epsilon-toxin production by Clostridium perfringens type D strain CN3718 is dependent upon the agr operon but not the VirS/VirR two-component regulatory system.mBio. 2011 Dec 13;2(6):e00275-11. doi: 10.1128/mBio.00275-11. Print 2011. mBio. 2011. Retraction in: mBio. 2022 Apr 26;13(2):e0049522. doi: 10.1128/mbio.00495-22. PMID: 22167225 Free PMC article. Retracted.
-
Evidence That VirS Is a Receptor for the Signaling Peptide of the Clostridium perfringens Agr-like Quorum Sensing System.mBio. 2020 Sep 15;11(5):e02219-20. doi: 10.1128/mBio.02219-20. mBio. 2020. PMID: 32934089 Free PMC article.
-
Host cell-induced signaling causes Clostridium perfringens to upregulate production of toxins important for intestinal infections.Gut Microbes. 2014 Jan-Feb;5(1):96-107. doi: 10.4161/gmic.26419. Epub 2013 Sep 10. Gut Microbes. 2014. PMID: 24061146 Free PMC article. Review.
-
The VirS/VirR two-component system regulates the anaerobic cytotoxicity, intestinal pathogenicity, and enterotoxemic lethality of Clostridium perfringens type C isolate CN3685.mBio. 2011 Jan 25;2(1):e00338-10. doi: 10.1128/mBio.00338-10. mBio. 2011. PMID: 21264065 Free PMC article.
-
Gene regulation by the VirS/VirR system in Clostridium perfringens.Anaerobe. 2016 Oct;41:5-9. doi: 10.1016/j.anaerobe.2016.06.003. Epub 2016 Jun 11. Anaerobe. 2016. PMID: 27296833 Review.
Cited by
-
Understanding Quorum-Sensing and Biofilm Forming in Anaerobic Bacterial Communities.Int J Mol Sci. 2024 Nov 28;25(23):12808. doi: 10.3390/ijms252312808. Int J Mol Sci. 2024. PMID: 39684519 Free PMC article. Review.
-
The presence of differentiated C2C12 muscle cells enhances toxin production and growth by Clostridium perfringens type A strain ATCC3624.Virulence. 2024 Dec;15(1):2388219. doi: 10.1080/21505594.2024.2388219. Epub 2024 Aug 27. Virulence. 2024. PMID: 39192628 Free PMC article.
References
-
- Rood JI. 2006. Clostridium perfringens and histotoxic disease, p 753–770. In Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (ed), The prokaryotes: a handbook on the biology of bacteria, 3rd ed, vol 4. Springer, New York, NY.
-
- McClane BA, Uzal FA, Miyakawa MF, Lyerly D, Wilkins TD. 2006. The enterotoxic Clostridia, p 688–752. In Dworkin MFalkow S, Rosenburg E, Schleifer H, Stackebrandt E (ed), The prokaryotes: a handbook on the biology of bacteria, 3rd ed. Springer, New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

