Spatial Organization of Chromatin: Emergence of Chromatin Structure During Development
- PMID: 34228506
- PMCID: PMC8664233
- DOI: 10.1146/annurev-cellbio-032321-035734
Spatial Organization of Chromatin: Emergence of Chromatin Structure During Development
Abstract
Nuclei are central hubs for information processing in eukaryotic cells. The need to fit large genomes into small nuclei imposes severe restrictions on genome organization and the mechanisms that drive genome-wide regulatory processes. How a disordered polymer such as chromatin, which has vast heterogeneity in its DNA and histone modification profiles, folds into discernibly consistent patterns is a fundamental question in biology. Outstanding questions include how genomes are spatially and temporally organized to regulate cellular processes with high precision and whether genome organization is causally linked to transcription regulation. The advent of next-generation sequencing, super-resolution imaging, multiplexed fluorescent in situ hybridization, and single-molecule imaging in individual living cells has caused a resurgence in efforts to understand the spatiotemporal organization of the genome. In this review, we discuss structural and mechanistic properties of genome organization at different length scales and examine changes in higher-order chromatin organization during important developmental transitions.
Keywords: chromosome topology; cohesin and condensin complexes; gametogenesis; loop extrusion; neural development; phase separation; zygotic genome activation.
Figures
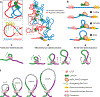
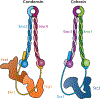



References
-
- Alberti S. 2017. Phase separation in biology. Curr. Biol 27(20):R1097–102 - PubMed

