SARS-CoV-2 neutralizing antibodies: Longevity, breadth, and evasion by emerging viral variants
- PMID: 34228725
- PMCID: PMC8291755
- DOI: 10.1371/journal.pmed.1003656
SARS-CoV-2 neutralizing antibodies: Longevity, breadth, and evasion by emerging viral variants
Abstract
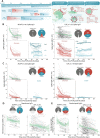
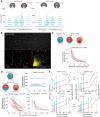
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) antibody neutralization response and its evasion by emerging viral variants and variant of concern (VOC) are unknown, but critical to understand reinfection risk and breakthrough infection following vaccination. Antibody immunoreactivity against SARS-CoV-2 antigens and Spike variants, inhibition of Spike-driven virus-cell fusion, and infectious SARS-CoV-2 neutralization were characterized in 807 serial samples from 233 reverse transcription polymerase chain reaction (RT-PCR)-confirmed Coronavirus Disease 2019 (COVID-19) individuals with detailed demographics and followed up to 7 months. A broad and sustained polyantigenic immunoreactivity against SARS-CoV-2 Spike, Membrane, and Nucleocapsid proteins, along with high viral neutralization, was associated with COVID-19 severity. A subgroup of "high responders" maintained high neutralizing responses over time, representing ideal convalescent plasma donors. Antibodies generated against SARS-CoV-2 during the first COVID-19 wave had reduced immunoreactivity and neutralization potency to emerging Spike variants and VOC. Accurate monitoring of SARS-CoV-2 antibody responses would be essential for selection of optimal responders and vaccine monitoring and design.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: FB has received honoraria from Biogen Idec and Merck Serono as invited speaker. The other authors have declared that no competing interests exist.
Figures



References
-
- Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–44. Epub 2020/07/10. doi: 10.1016/S0140-6736(20)31483-5 ; PubMed Central PMCID: PMC7336131. - DOI - PMC - PubMed
-
- Rogers TF, Zhao F, Huang D, Beutler N, Burns A, He WT, et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369(6506):956–63. Epub 2020/06/17. doi: 10.1126/science.abc7520 ; PubMed Central PMCID: PMC7299280. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

