Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis
- PMID: 34228774
- PMCID: PMC8261689
- DOI: 10.1001/jama.2021.11330
Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis
Abstract
Importance: Clinical trials assessing the efficacy of IL-6 antagonists in patients hospitalized for COVID-19 have variously reported benefit, no effect, and harm.
Objective: To estimate the association between administration of IL-6 antagonists compared with usual care or placebo and 28-day all-cause mortality and other outcomes.
Data sources: Trials were identified through systematic searches of electronic databases between October 2020 and January 2021. Searches were not restricted by trial status or language. Additional trials were identified through contact with experts.
Study selection: Eligible trials randomly assigned patients hospitalized for COVID-19 to a group in whom IL-6 antagonists were administered and to a group in whom neither IL-6 antagonists nor any other immunomodulators except corticosteroids were administered. Among 72 potentially eligible trials, 27 (37.5%) met study selection criteria.
Data extraction and synthesis: In this prospective meta-analysis, risk of bias was assessed using the Cochrane Risk of Bias Assessment Tool. Inconsistency among trial results was assessed using the I2 statistic. The primary analysis was an inverse variance-weighted fixed-effects meta-analysis of odds ratios (ORs) for 28-day all-cause mortality.
Main outcomes and measures: The primary outcome measure was all-cause mortality at 28 days after randomization. There were 9 secondary outcomes including progression to invasive mechanical ventilation or death and risk of secondary infection by 28 days.
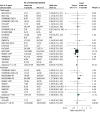
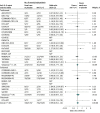
Results: A total of 10 930 patients (median age, 61 years [range of medians, 52-68 years]; 3560 [33%] were women) participating in 27 trials were included. By 28 days, there were 1407 deaths among 6449 patients randomized to IL-6 antagonists and 1158 deaths among 4481 patients randomized to usual care or placebo (summary OR, 0.86 [95% CI, 0.79-0.95]; P = .003 based on a fixed-effects meta-analysis). This corresponds to an absolute mortality risk of 22% for IL-6 antagonists compared with an assumed mortality risk of 25% for usual care or placebo. The corresponding summary ORs were 0.83 (95% CI, 0.74-0.92; P < .001) for tocilizumab and 1.08 (95% CI, 0.86-1.36; P = .52) for sarilumab. The summary ORs for the association with mortality compared with usual care or placebo in those receiving corticosteroids were 0.77 (95% CI, 0.68-0.87) for tocilizumab and 0.92 (95% CI, 0.61-1.38) for sarilumab. The ORs for the association with progression to invasive mechanical ventilation or death, compared with usual care or placebo, were 0.77 (95% CI, 0.70-0.85) for all IL-6 antagonists, 0.74 (95% CI, 0.66-0.82) for tocilizumab, and 1.00 (95% CI, 0.74-1.34) for sarilumab. Secondary infections by 28 days occurred in 21.9% of patients treated with IL-6 antagonists vs 17.6% of patients treated with usual care or placebo (OR accounting for trial sample sizes, 0.99; 95% CI, 0.85-1.16).
Conclusions and relevance: In this prospective meta-analysis of clinical trials of patients hospitalized for COVID-19, administration of IL-6 antagonists, compared with usual care or placebo, was associated with lower 28-day all-cause mortality.
Trial registration: PROSPERO Identifier: CRD42021230155.
Conflict of interest statement
Figures


Comment in
-
IL-6 Receptor Antagonist Therapy for Patients Hospitalized for COVID-19: Who, When, and How?JAMA. 2021 Aug 10;326(6):483-485. doi: 10.1001/jama.2021.11121. JAMA. 2021. PMID: 34228779 No abstract available.
-
In patients hospitalized for COVID-19, tocilizumab reduces mortality at 28 d.Ann Intern Med. 2021 Nov;174(11):JC125. doi: 10.7326/ACPJ202111160-125. Epub 2021 Nov 2. Ann Intern Med. 2021. PMID: 34724398
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

