Non-invasive duo positive airway pressure ventilation versus nasal continuous positive airway pressure in preterm infants with respiratory distress syndrome: a randomized controlled trial
- PMID: 34229655
- PMCID: PMC8259388
- DOI: 10.1186/s12887-021-02741-w
Non-invasive duo positive airway pressure ventilation versus nasal continuous positive airway pressure in preterm infants with respiratory distress syndrome: a randomized controlled trial
Abstract
Background: The most common cause of respiratory failure in premature infants is respiratory distress syndrome. Historically, respiratory distress syndrome has been treated by intratracheal surfactant injection followed by mechanical ventilation. In view of the risk of pulmonary injury associated with mechanical ventilation and subsequent chronic pulmonary lung disease, less invasive treatment modalities have been suggested to reduce pulmonary complications.
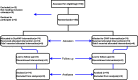
Methods: 148 neonates (with gestational age of 28 to 34 weeks) with respiratory distress syndrome admitted to Imam Khomeini Hospital in Ahwaz in 2018 were enrolled in this clinical trial study. 74 neonates were assigned to duo positive airway pressure (NDUOPAP) group and 74 neonates to nasal continuous positive airway pressure (NCPAP) group. The primary outcome in this study was failure of N-DUOPAP and NCPAP treatments within the first 72 h after birth and secondary outcomes included treatment complications.
Results: there was not significant difference between DUOPAP (4.1 %) and NCPAP (8.1 %) in treatment failure at the first 72 h of birth (p = 0.494), but non-invasive ventilation time was less in the DUOPAP group (p = 0.004). There were not significant differences in the frequency of patent ductus arteriosus (PDA), pneumothorax, intraventricular hemorrhage (IVH) and bronchopulmonary dysplasia (BPD), apnea and mortality between the two groups. Need for repeated doses of surfactant (p = 0.042) in the NDUOPAP group was significantly lower than that of the NCPAP group. The duration of oxygen therapy in the NDUOPAP group was significantly lower than that of the NCPAP group (p = 0.034). Also, the duration of hospitalization in the NDUOPAP group was shorter than that of the NCPAP group (p = 0.002).
Conclusions: In the present study, DUOPAP compared to NCPAP did not reduce the need for mechanical ventilation during the first 72 h of birth, but the duration of non-invasive ventilation and oxygen demand, the need for multiple doses of surfactant and length of stay in the DUOPAP group were less than those in the CPAP group.
Trial registration: IRCT20180821040847N1 , Approved on 2018-09-10.
Keywords: Duo positive airway pressure; Nasal continuous positive airway pressure; Preterm infants; Respiratory distress syndrome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Gnanaratnem J, Finer N. Neonatal acute repiratory failure. Curr opin pedicutr. 2000 Jun;12(13):227 – 32. 10.1097/00008480-200006000-00009. - PubMed
-
- Reiterer F, Polin RA. Non –invasive ventilation in Preterm infants: a clinical Review. Int J Pediatr Neonat Care. 2016;2:118. doi: 10.15344/2455-2364/2016/118. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources