Chronic colitis exacerbates NLRP3-dependent neuroinflammation and cognitive impairment in middle-aged brain
- PMID: 34229722
- PMCID: PMC8262017
- DOI: 10.1186/s12974-021-02199-8
Chronic colitis exacerbates NLRP3-dependent neuroinflammation and cognitive impairment in middle-aged brain
Erratum in
-
Correction to: Chronic colitis exacerbates NLRP3-dependent neuroinflammation and cognitive impairment in middle-aged brain.J Neuroinflammation. 2022 Jan 15;19(1):18. doi: 10.1186/s12974-021-02347-0. J Neuroinflammation. 2022. PMID: 35033113 Free PMC article. No abstract available.
Abstract
Background: Neuroinflammation is a major driver of age-related brain degeneration and concomitant functional impairment. In patients with Alzheimer's disease, the most common form of age-related dementia, factors that enhance neuroinflammation may exacerbate disease progression, in part by impairing the glymphatic system responsible for clearance of pathogenic beta-amyloid. Inflammatory bowel diseases (IBDs) induce neuroinflammation and exacerbate cognitive impairment in the elderly. The NACHT-LRR and pyrin (PYD) domain-containing protein 3 (NLRP3) inflammasome has been implicated in neuroinflammation. Therefore, we examined if the NLRP3 inflammasome contributes to glymphatic dysfunction and cognitive impairment in an aging mouse model of IBD.
Methods: Sixteen-month-old C57BL/6J and NLRP3 knockout (KO) mice received 1% wt/vol dextran sodium sulfate (DSS) in drinking water to model IBD. Colitis induction was confirmed by histopathology. Exploratory behavior was examined in the open field, associative memory by the novel-object recognition and Morris water maze tests, glymphatic clearance by in vivo two-photon imaging, and neuroinflammation by immunofluorescence and western blotting detection of inflammatory markers.
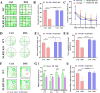
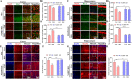
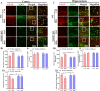
Results: Administration of DSS induced colitis, impaired spatial and recognition memory, activated microglia, and increased A1-like astrocyte numbers. In addition, DSS treatment impaired glymphatic clearance, aggravated amyloid plaque accumulation, and induced neuronal loss in the cortex and hippocampus. These neurodegenerative responses were associated with increased NLRP3 inflammasome expression and accumulation of gut-derived T lymphocytes along meningeal lymphatic vessels. Conversely, NLRP3 depletion protected against cognitive dysfunction, neuroinflammation, and neurological damage induced by DSS.
Conclusions: Colitis can exacerbate age-related neuropathology, while suppression of NLRP3 inflammasome activity may protect against these deleterious effects of colitis.
Keywords: Cognition; Glymphatic clearance; Inflammatory bowel disease; NLRP3 inflammasome; T cell.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
MeSH terms
Substances
Grants and funding
- 81902285, 81871847, 81672261, 81972151, 8157228, 81873751/Grants from the National Natural Science Foundation of China
- 2019A1515011444, 2017A030313493, 2019A1515011106/Guangdong Basic and Applied Basic Research Foundation
- 201803010119/the Science and Technology Planning Key Project of Guangzhou, China
- 201604020009/Guangzhou health and medical collaborative innovation major projects
- 2017B030314103/the Guangdong Provincial Key Laboratory for Diagnosis and Treatment of Major Neurological Diseases
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

