Efficacy of a Low Dose of Melatonin as an Adjunctive Therapy in Hospitalized Patients with COVID-19: A Randomized, Double-blind Clinical Trial
- PMID: 34229896
- PMCID: PMC8220995
- DOI: 10.1016/j.arcmed.2021.06.006
Efficacy of a Low Dose of Melatonin as an Adjunctive Therapy in Hospitalized Patients with COVID-19: A Randomized, Double-blind Clinical Trial
Abstract
Background: Melatonin has been known as an anti-inflammatory agent and immune modulator that may address progressive pathophysiology of coronavirus disease 2019 (COVID-19).
Aim of the study: To evaluate the clinical efficacy of adjuvant, use of melatonin in patients with COVID-19.
Methods: This single-center, double-blind, randomized clinical trial included 74 hospitalized patients with confirmed mild to moderate COVID-19 at Baqiyatallah Hospital in Tehran, Iran, from April 25, 2020-June 5, 2020. Patients were randomly assigned in a 1:1 ratio to receive standard of care and standard of care plus melatonin at a dose of 3 mg three times daily for 14 d. Clinical characteristics, laboratory, and radiological findings were assessed and compared between two study groups at baseline and post-intervention. Safety and clinical outcomes were followed up for four weeks.
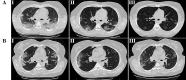
Results: A total of 24 patients in the intervention group and 20 patients in the control group completed the treatment. Compared with the control group, the clinical symptoms such as cough, dyspnea, and fatigue, as well as the level of CRP and the pulmonary involvement in the intervention group had significantly improved (p <0.05). The mean time of hospital discharge of patients and return to baseline health was significantly shorter in the intervention group compared to the control group (p <0.05). No deaths and adverse events were observed in both groups.
Conclusions: Adjuvant use of melatonin has a potential to improve clinical symptoms of COVID-19 patients and contribute to a faster return of patients to baseline health.
Keywords: Adjunctive therapy; COVID-19; Clinical trial; Melatonin.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Competing Interests All authors have no conflict of interest to declare.
Figures
References
-
- World Health Organization. Weekly epidemiological update on COVID-19. https://www.who.int/publications/m/item/weekly-epidemiological-update-on.... (Accessed May 4, 2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

