Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines
- PMID: 34230920
- PMCID: PMC8248717
- DOI: 10.1016/j.medj.2021.06.006
Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines
Abstract
Background: As the coronavirus disease 2019 (COVID-19) vaccination campaign unfolds, it is important to continuously assess the real-world safety of Food and Drug Administration (FDA)-authorized vaccines. Curation of large-scale electronic health records (EHRs) enables near-real-time safety evaluations that were not previously possible.
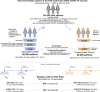
Methods: In this retrospective study, we deployed deep neural networks over a large EHR system to automatically curate the adverse effects mentioned by physicians in over 1.2 million clinical notes between December 1, 2020 and April 20, 2021. We compared notes from 68,266 individuals who received at least one dose of BNT162b2 (n = 51,795) or mRNA-1273 (n = 16,471) to notes from 68,266 unvaccinated individuals who were matched by demographic, geographic, and clinical features.
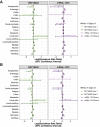
Findings: Individuals vaccinated with BNT162b2 or mRNA-1273 had a higher rate of return to the clinic, but not the emergency department, after both doses compared to unvaccinated controls. The most frequently documented adverse effects within 7 days of each vaccine dose included myalgia, headache, and fatigue, but the rates of EHR documentation for each side effect were remarkably low compared to those derived from active solicitation during clinical trials. Severe events, including anaphylaxis, facial paralysis, and cerebral venous sinus thrombosis, were rare and occurred at similar frequencies in vaccinated and unvaccinated individuals.
Conclusions: This analysis of vaccine-related adverse effects from over 1.2 million EHR notes of more than 130,000 individuals reaffirms the safety and tolerability of the FDA-authorized mRNA COVID-19 vaccines in practice.
Funding: This study was funded by nference.
Keywords: BNT162b2; COVID-19; COVID-19 vaccines; mRNA-1273; propensity score matching; real world analysis; vaccine safety.
© 2021 Elsevier Inc.
Conflict of interest statement
R.M., P.L., E.S., A.P., S.A., C.P., V.A., A.J.V., P.A., A.R., C.C., K.C., D.D., N.K., E.R., G.B., A.M., T.W., and V.S. are employees of nference and have financial interests in the company and in the successful application of this research. R.M. is a student at Boston University School of Medicine. P.L. is a student at Harvard Medical School. J.C.O. receives personal fees from Elsevier and Bates College and small grants from nference outside the submitted work. A.D.B. is a consultant for AbbVie, is on scientific advisory boards for nference and Zentalis, and is founder and president of Splissen Therapeutics. J.H., J.C.O., G.J.G., A.W.W., A.V., M.D.S., and A.D.B. are employees of the Mayo Clinic. The Mayo Clinic may stand to gain financially from the successful outcome of the research. nference collaborates with Janssen and other bio-pharmaceutical companies on data science initiatives unrelated to this study. These collaborations had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic conflict-of-interest policies.
Figures


References
-
- Centers for Disease Control and Prevention (CDC) 2020. COVID Data Tracker.https://covid.cdc.gov/covid-data-tracker/
-
- FDA authorizes Moderna COVID-19 vaccine. Med. Lett. Drugs Ther. 2021;63:9–10. - PubMed
-
- Office of the Commissioner . 2021. Pfizer-BioNTech COVID-19 Vaccine.https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

