This is a preprint.
Combining genomic and epidemiological data to compare the transmissibility of SARS-CoV-2 lineages
- PMID: 34230938
- PMCID: PMC8259915
- DOI: 10.1101/2021.07.01.21259859
Combining genomic and epidemiological data to compare the transmissibility of SARS-CoV-2 lineages
Update in
-
Combining genomic and epidemiological data to compare the transmissibility of SARS-CoV-2 variants Alpha and Iota.Commun Biol. 2022 May 11;5(1):439. doi: 10.1038/s42003-022-03347-3. Commun Biol. 2022. PMID: 35545661 Free PMC article.
Abstract
Emerging SARS-CoV-2 variants have shaped the second year of the COVID-19 pandemic and the public health discourse around effective control measures. Evaluating the public health threat posed by a new variant is essential for appropriately adapting response efforts when community transmission is detected. However, this assessment requires that a true comparison can be made between the new variant and its predecessors because factors other than the virus genotype may influence spread and transmission. In this study, we develop a framework that integrates genomic surveillance data to estimate the relative effective reproduction number (R t ) of co-circulating lineages. We use Connecticut, a state in the northeastern United States in which the SARS-CoV-2 variants B.1.1.7 and B.1.526 co-circulated in early 2021, as a case study for implementing this framework. We find that the R t of B.1.1.7 was 6-10% larger than that of B.1.526 in Connecticut in the midst of a COVID-19 vaccination campaign. To assess the generalizability of this framework, we apply it to genomic surveillance data from New York City and observe the same trend. Finally, we use discrete phylogeography to demonstrate that while both variants were introduced into Connecticut at comparable frequencies, clades that resulted from introductions of B.1.1.7 were larger than those resulting from B.1.526 introductions. Our framework, which uses open-source methods requiring minimal computational resources, may be used to monitor near real-time variant dynamics in a myriad of settings.
Figures

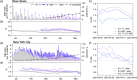

References
-
- Faria N. R. et al. Genomic characterisation of an emergent SARS-CoV-2 lineage in Manaus: preliminary findings. Virological (2021).
-
- Rambaut Andrew, Loman Nick, Pybus Oliver, Barclay Wendy, Barrett Jeff, Carabelli Alesandro, Connor Tom, Peacock Tom, David L Robertson Erik Volz. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. Virological (2020).
-
- Wibmer C. K. et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 27, 622–625 (2021). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
