18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Following Chimeric Antigen Receptor T-cell Therapy in Large B-cell Lymphoma
- PMID: 34231105
- PMCID: PMC8578305
- DOI: 10.1007/s11307-021-01627-8
18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Following Chimeric Antigen Receptor T-cell Therapy in Large B-cell Lymphoma
Abstract
Purpose: 18F-Fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) is a well-established imaging modality to assess responses in patients with B-cell neoplasms. However, there is limited information about the utility of FDG PET/CT after chimeric antigen receptor T-cell (CART) therapies for large B-cell lymphomas. In this retrospective analysis, we aimed to evaluate how FDG PET/CT performs in patients receiving commercially available anti-CD19 CART therapies for relapsed/refractory (r/r) large B-cell lymphomas. In addition, we examined the time to repeat scan and the rate of pseudoprogression within this population. Lastly, the rates of radiographic response to CART therapy using FDG PET/CT are reported.
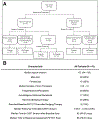
Procedures: The pre-treatment and post-treatment scans were analyzed from a selected cohort of 43 patients from a single institution. Patients were stratified by diagnosis of either a first occurrence of diffuse large B-cell lymphoma: de novo diffuse large B-cell lymphoma (DLBCL); or a transformed diffuse large B-cell lymphoma arising from indolent non-Hodgkin lymphoma (t-iNHL).
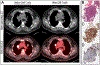
Results: More patients received CART therapy for DLBCL than t-iNHL (65 % vs 35 %). FDG PET/CT had a 99 % sensitivity and 100 % specificity for detecting recurrent disease in this group. The median time to initial response assessment was 86 days (IQR 79-91; full range 24-146) after infusion. There were no biopsy-proven cases of pseudoprogression identified. In this selected group of patients, the overall response rate by Lugano 2014 criteria was 56 %. All patients with a partial response (N = 6) eventually progressed despite additional therapy.
Conclusions: Due to its excellent test characteristics and ability to detect asymptomatic disease, routine surveillance with PET/CT at 3 months after CART infusion is supported by our data. Earlier PET/CT may be of value in select situations as we did not find any cases of pseudoprogression.
Keywords: Chimeric antigen receptor T-cell; FDG PET/CT; Immune imaging; Lymphomas; Reporter gene imaging.
© 2021. World Molecular Imaging Society.
Figures

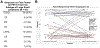

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

