Enhanced Ability of Plant-Derived PGT121 Glycovariants To Eliminate HIV-1-Infected Cells
- PMID: 34232070
- PMCID: PMC8387047
- DOI: 10.1128/JVI.00796-21
Enhanced Ability of Plant-Derived PGT121 Glycovariants To Eliminate HIV-1-Infected Cells
Abstract
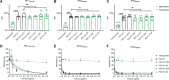
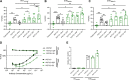
The activity of broadly neutralizing antibodies (bNAbs) targeting HIV-1 depends on pleiotropic functions, including viral neutralization and the elimination of HIV-1-infected cells. Several in vivo studies have suggested that passive administration of bNAbs represents a valuable strategy for the prevention or treatment of HIV-1. In addition, different strategies are currently being tested to scale up the production of bNAbs to obtain the large quantities of antibodies required for clinical trials. Production of antibodies in plants permits low-cost and large-scale production of valuable therapeutics; furthermore, pertinent to this work, it also includes an advanced glycoengineering platform. In this study, we used Nicotiana benthamiana to produce different Fc-glycovariants of a potent bNAb, PGT121, with near-homogeneous profiles and evaluated their antiviral activities. Structural analyses identified a close similarity in overall structure and glycosylation patterns of Fc regions for these plant-derived Abs and mammalian cell-derived Abs. When tested for Fc-effector activities, afucosylated PGT121 showed significantly enhanced FcγRIIIa interaction and antibody dependent cellular cytotoxicity (ADCC) against primary HIV-1-infected cells, both in vitro and ex vivo. However, the overall galactosylation profiles of plant PGT121 did not affect ADCC activities against infected primary CD4+ T cells. Our results suggest that the abrogation of the Fc N-linked glycan fucosylation of PGT121 is a worthwhile strategy to boost its Fc-effector functionality. IMPORTANCE PGT121 is a highly potent bNAb and its antiviral activities for HIV-1 prevention and therapy are currently being evaluated in clinical trials. The importance of its Fc-effector functions in clearing HIV-1-infected cells is also under investigation. Our results highlight enhanced Fc-effector activities of afucosylated PGT121 MAbs that could be important in a therapeutic context to accelerate infected cell clearance and slow disease progression. Future studies to evaluate the potential of plant-produced afucosylated PGT121 in controlling HIV-1 replication in vivo are warranted.
Keywords: ADCC; Env glycoproteins; Envelope glycoproteins; FcγRIIIa; HIV-1; Nicotiana benthamiana; PGT121; broadly neutralizing antibodies; fucose; galactose; glycosylation; neutralizing antibodies; plant antibodies.
Figures





References
-
- Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR. 2013. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503:224–228. 10.1038/nature12744. - DOI - PMC - PubMed
-
- Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, Malbec M, Gravemann S, Billerbeck E, Dorner M, Buning H, Schwartz O, Knops E, Kaiser R, Seaman MS, Wilson JM, Rice CM, Ploss A, Bjorkman PJ, Klein F, Nussenzweig MC. 2013. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A 110:16538–16543. 10.1073/pnas.1315295110. - DOI - PMC - PubMed
-
- Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio P, Incesu RB, Eisenreich TR, Bieniasz PD, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. 2012. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492:118–122. 10.1038/nature11604. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 GM133893/GM/NIGMS NIH HHS/United States
- R01 AI148379/AI/NIAID NIH HHS/United States
- P01 AI150471/AI/NIAID NIH HHS/United States
- R01 AI129769/AI/NIAID NIH HHS/United States
- R01 AI136621/AI/NIAID NIH HHS/United States
- P01 AI120756/AI/NIAID NIH HHS/United States
- R01 AI121135/AI/NIAID NIH HHS/United States
- R01 AI155163/AI/NIAID NIH HHS/United States
- R01 AI116274/AI/NIAID NIH HHS/United States
- R37 AI095098/AI/NIAID NIH HHS/United States
- UM1 AI144462/AI/NIAID NIH HHS/United States
- R01 AI161816/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

