Single molecule analysis indicates stimulation of MUTYH by UV-DDB through enzyme turnover
- PMID: 34232996
- PMCID: PMC8373069
- DOI: 10.1093/nar/gkab591
Single molecule analysis indicates stimulation of MUTYH by UV-DDB through enzyme turnover
Abstract
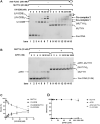
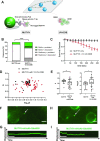
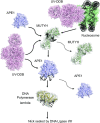
The oxidative base damage, 8-oxo-7,8-dihydroguanine (8-oxoG) is a highly mutagenic lesion because replicative DNA polymerases insert adenine (A) opposite 8-oxoG. In mammalian cells, the removal of A incorporated across from 8-oxoG is mediated by the glycosylase MUTYH during base excision repair (BER). After A excision, MUTYH binds avidly to the abasic site and is thus product inhibited. We have previously reported that UV-DDB plays a non-canonical role in BER during the removal of 8-oxoG by 8-oxoG glycosylase, OGG1 and presented preliminary data that UV-DDB can also increase MUTYH activity. In this present study we examine the mechanism of how UV-DDB stimulates MUTYH. Bulk kinetic assays show that UV-DDB can stimulate the turnover rate of MUTYH excision of A across from 8-oxoG by 4-5-fold. Electrophoretic mobility shift assays and atomic force microscopy suggest transient complex formation between MUTYH and UV-DDB, which displaces MUTYH from abasic sites. Using single molecule fluorescence analysis of MUTYH bound to abasic sites, we show that UV-DDB interacts directly with MUTYH and increases the mobility and dissociation rate of MUTYH. UV-DDB decreases MUTYH half-life on abasic sites in DNA from 8800 to 590 seconds. Together these data suggest that UV-DDB facilitates productive turnover of MUTYH at abasic sites during 8-oxoG:A repair.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Gu Y., Parker A., Wilson T.M., Bai H., Chang D.Y., Lu A.L.. Human MutY homolog, a DNA glycosylase involved in base excision repair, physically and functionally interacts with mismatch repair proteins human MutS homolog 2/human MutS homolog 6. J. Biol. Chem. 2002; 277:11135–11142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

