GABA-receptive microglia selectively sculpt developing inhibitory circuits
- PMID: 34233165
- PMCID: PMC9122259
- DOI: 10.1016/j.cell.2021.06.018
GABA-receptive microglia selectively sculpt developing inhibitory circuits
Erratum in
-
GABA-receptive microglia selectively sculpt developing inhibitory circuits.Cell. 2021 Oct 28;184(22):5686. doi: 10.1016/j.cell.2021.10.009. Cell. 2021. PMID: 34715023 Free PMC article. No abstract available.
Abstract
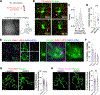
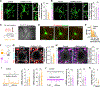
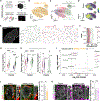
Microglia, the resident immune cells of the brain, have emerged as crucial regulators of synaptic refinement and brain wiring. However, whether the remodeling of distinct synapse types during development is mediated by specialized microglia is unknown. Here, we show that GABA-receptive microglia selectively interact with inhibitory cortical synapses during a critical window of mouse postnatal development. GABA initiates a transcriptional synapse remodeling program within these specialized microglia, which in turn sculpt inhibitory connectivity without impacting excitatory synapses. Ablation of GABAB receptors within microglia impairs this process and leads to behavioral abnormalities. These findings demonstrate that brain wiring relies on the selective communication between matched neuronal and glial cell types.
Keywords: cerebral cortex; development; glia-neuron interactions; inhibitory synapses; interneurons; microglia; neurodevelopmental disorders.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests B.S. serves on the SAB and is a minor shareholder of Annexon. G.F. is a founder of Regel. The other authors declare no competing interests.
Figures




Comment in
-
Microglia prune inhibitory synapses, too.Nat Rev Neurosci. 2021 Sep;22(9):517. doi: 10.1038/s41583-021-00504-1. Nat Rev Neurosci. 2021. PMID: 34312535 No abstract available.
References
-
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (APA Publishing; ).
-
- Bohlen CJ, Friedman BA, Dejanovic B, and Sheng M. (2019). Microglia in Brain Development, Homeostasis, and Neurodegeneration. Annu. Rev. Genet 53, 263–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

