Acceleration of age-induced proteolysis in the guinea pig lens nucleus by in vivo exposure to hyperbaric oxygen: A mass spectrometry analysis
- PMID: 34233175
- PMCID: PMC8429224
- DOI: 10.1016/j.exer.2021.108697
Acceleration of age-induced proteolysis in the guinea pig lens nucleus by in vivo exposure to hyperbaric oxygen: A mass spectrometry analysis
Abstract
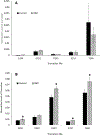
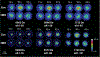
Hyperbaric oxygen (HBO) treatment of animals or ocular lenses in culture recapitulates many molecular changes observed in human age-related nuclear cataract. The guinea pig HBO model has been one of the best examples of such treatment leading to dose-dependent development of lens nuclear opacities. In this study, complimentary mass spectrometry methods were employed to examine protein truncation after HBO treatment of aged guinea pigs. Quantitative liquid chromatography-mass spectrometry (LC-MS) analysis of the membrane fraction of guinea pig lenses showed statistically significant increases in aquaporin-0 (AQP0) C-terminal truncation, consistent with previous reports of accelerated loss of membrane and cytoskeletal proteins. In addition, imaging mass spectrometry (IMS) analysis spatially mapped the acceleration of age-related αA-crystallin truncation in the lens nucleus. The truncation sites in αA-crystallin closely match those observed in human lenses with age. Taken together, our results suggest that HBO accelerates the normal lens aging process and leads to nuclear cataract.
Keywords: Alpha crystallin; Aquaporin-0; Hyperbaric oxygen; Lens; Mass spectrometry; Nuclear cataract; Protein truncation; αA66-80 peptide.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures



References
-
- Anderson DM, Floyd KA, Barnes S, Clark JM, Clark JI, Mchaourab H and Schey KL, 2015. A method to prevent protein delocalization in imaging mass spectrometry of non-adherent tissues: application to small vertebrate lens imaging. Anal Bioanal Chem. 407, 2311–2320. 10.1007/s00216-015-8489-5 - DOI - PMC - PubMed
-
- Borchman D, Giblin FJ, Leverenz VR, Reddy VN, Lin LR, Yappert MC, Tang D and Li L, 2000. Impact of aging and hyperbaric oxygen in vivo on guinea pig lens lipids and nuclear light scatter. Invest Ophthalmol Vis Sci. 41, 3061–3073. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

