Identification of a class of non-conventional ER-stress-response-derived immunogenic peptides
- PMID: 34233181
- PMCID: PMC8278487
- DOI: 10.1016/j.celrep.2021.109312
Identification of a class of non-conventional ER-stress-response-derived immunogenic peptides
Abstract
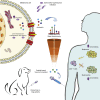
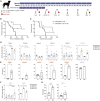
Efforts to overcome resistance to immune checkpoint blockade therapy have focused on vaccination strategies using neoepitopes, although they cannot be applied on a large scale due to the "private" nature of cancer mutations. Here, we show that infection of tumor cells with Salmonella induces the opening of membrane hemichannels and the extracellular release of proteasome-generated peptides by the exacerbation of endoplasmic reticulum (ER) stress. Peptides released by cancer cells foster an antitumor response in vivo, both in mice bearing B16F10 melanomas and in dogs suffering from osteosarcoma. Mass spectrometry analysis on the supernatant of human melanoma cells revealed 12 peptides capable of priming healthy-donor CD8+ T cells that recognize and kill human melanoma cells in vitro and when xenotransplanted in vivo. Hence, we identified a class of shared tumor antigens that are generated in ER-stressed cells, such as tumor cells, that do not induce tolerance and are not presented by healthy cells.
Keywords: ER-stress response; Salmonella; cancer; immunotherapy; tumor antigens; vaccine.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Bartoszewski R., Brewer J.W., Rab A., Crossman D.K., Bartoszewska S., Kapoor N., Fuller C., Collawn J.F., Bebok Z. The unfolded protein response (UPR)-activated transcription factor X-box-binding protein 1 (XBP1) induces microRNA-346 expression that targets the human antigen peptide transporter 1 (TAP1) mRNA and governs immune regulatory genes. J. Biol. Chem. 2011;286:41862–41870. - PMC - PubMed
-
- Cerezo M., Lehraiki A., Millet A., Rouaud F., Plaisant M., Jaune E., Botton T., Ronco C., Abbe P., Amdouni H. Compounds triggering ER stress exert anti- melanoma effects and overcome BRAF inhibitor resistance. Cancer Cell. 2016;29:805–819. - PubMed
-
- Chapiro J., Claverol S., Piette F., Ma W., Stroobant V., Guillaume B., Gairin J.E., Morel S., Burlet-Schiltz O., Monsarrat B. Destructive cleavage of antigenic peptides either by the immunoproteasome or by the standard proteasome results in differential antigen presentation. J. Immunol. 2006;176:1053–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

