Structure of the TELO2-TTI1-TTI2 complex and its function in TOR recruitment to the R2TP chaperone
- PMID: 34233195
- PMCID: PMC8278493
- DOI: 10.1016/j.celrep.2021.109317
Structure of the TELO2-TTI1-TTI2 complex and its function in TOR recruitment to the R2TP chaperone
Abstract
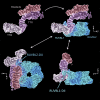
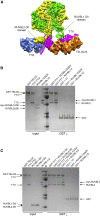
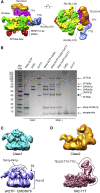
The R2TP (RUVBL1-RUVBL2-RPAP3-PIH1D1) complex, in collaboration with heat shock protein 90 (HSP90), functions as a chaperone for the assembly and stability of protein complexes, including RNA polymerases, small nuclear ribonucleoprotein particles (snRNPs), and phosphatidylinositol 3-kinase (PI3K)-like kinases (PIKKs) such as TOR and SMG1. PIKK stabilization depends on an additional complex of TELO2, TTI1, and TTI2 (TTT), whose structure and function are poorly understood. The cryoelectron microscopy (cryo-EM) structure of the human R2TP-TTT complex, together with biochemical experiments, reveals the mechanism of TOR recruitment to the R2TP-TTT chaperone. The HEAT-repeat TTT complex binds the kinase domain of TOR, without blocking its activity, and delivers TOR to the R2TP chaperone. In addition, TTT regulates the R2TP chaperone by inhibiting RUVBL1-RUVBL2 ATPase activity and by modulating the conformation and interactions of the PIH1D1 and RPAP3 components of R2TP. Taken together, our results show how TTT couples the recruitment of TOR to R2TP with the regulation of this chaperone system.
Keywords: HSP90 chaperone; PIKK; R2TP; RUVBL1; RUVBL2; TELO2; TTI1; TTI2; TTT; mTOR.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Similar articles
-
Structure of the Human TELO2-TTI1-TTI2 Complex.J Mol Biol. 2022 Jan 30;434(2):167370. doi: 10.1016/j.jmb.2021.167370. Epub 2021 Nov 24. J Mol Biol. 2022. PMID: 34838521
-
Advances on the Structure of the R2TP/Prefoldin-like Complex.Adv Exp Med Biol. 2018;1106:73-83. doi: 10.1007/978-3-030-00737-9_5. Adv Exp Med Biol. 2018. PMID: 30484153
-
RPAP3 C-Terminal Domain: A Conserved Domain for the Assembly of R2TP Co-Chaperone Complexes.Cells. 2020 May 6;9(5):1139. doi: 10.3390/cells9051139. Cells. 2020. PMID: 32384603 Free PMC article. Review.
-
Maturation and Assembly of mTOR Complexes by the HSP90-R2TP-TTT Chaperone System: Molecular Insights and Mechanisms.Subcell Biochem. 2024;104:459-483. doi: 10.1007/978-3-031-58843-3_17. Subcell Biochem. 2024. PMID: 38963496 Review.
-
Structural basis for phosphorylation-dependent recruitment of Tel2 to Hsp90 by Pih1.Structure. 2014 Jun 10;22(6):805-18. doi: 10.1016/j.str.2014.04.001. Epub 2014 May 1. Structure. 2014. PMID: 24794838 Free PMC article.
Cited by
-
Exploring the Functional Roles of Telomere Maintenance 2 in the Tumorigenesis of Glioblastoma Multiforme and Drug Responsiveness to Temozolomide.Int J Mol Sci. 2023 May 25;24(11):9256. doi: 10.3390/ijms24119256. Int J Mol Sci. 2023. PMID: 37298208 Free PMC article.
-
The Role of Hsp90-R2TP in Macromolecular Complex Assembly and Stabilization.Biomolecules. 2022 Jul 28;12(8):1045. doi: 10.3390/biom12081045. Biomolecules. 2022. PMID: 36008939 Free PMC article. Review.
-
TTT (Tel2-Tti1-Tti2) Complex, the Co-Chaperone of PIKKs and a Potential Target for Cancer Chemotherapy.Int J Mol Sci. 2023 May 5;24(9):8268. doi: 10.3390/ijms24098268. Int J Mol Sci. 2023. PMID: 37175973 Free PMC article. Review.
-
First Virtual International Congress on Cellular and Organismal Stress Responses, November 5-6, 2020.Cell Stress Chaperones. 2021 Mar;26(2):289-295. doi: 10.1007/s12192-021-01192-7. Epub 2021 Feb 9. Cell Stress Chaperones. 2021. PMID: 33559835 Free PMC article. Review.
-
TTI1 promotes non-small-cell lung cancer progression by regulating the mTOR signaling pathway.Cancer Sci. 2023 Mar;114(3):855-869. doi: 10.1111/cas.15668. Epub 2022 Dec 7. Cancer Sci. 2023. PMID: 36403197 Free PMC article.
References
-
- Emsley P., Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

