Binge ethanol drinking associated with sex-dependent plasticity of neurons in the insula that project to the bed nucleus of the stria terminalis
- PMID: 34233202
- PMCID: PMC8928450
- DOI: 10.1016/j.neuropharm.2021.108695
Binge ethanol drinking associated with sex-dependent plasticity of neurons in the insula that project to the bed nucleus of the stria terminalis
Abstract
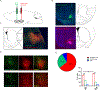
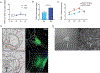
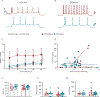
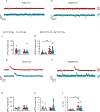
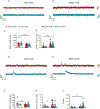
Modifications in brain regions that govern reward-seeking are thought to contribute to persistent behaviors that are heavily associated with alcohol-use disorder (AUD) including binge ethanol drinking. The bed nucleus of the stria terminalis (BNST) is a critical node linked to both alcohol consumption and the onset, maintenance and progression of adaptive anxiety and stress-related disorders. Differences in anatomy, connectivity and receptor subpopulations, make the BNST a sexually dimorphic region. Previous work indicates that the ventral BNST (vBNST) receives input from the insular cortex (IC), a brain region involved in processing the body's internal state. This IC-vBNST projection has also been implicated in emotional and reward-seeking processes. Therefore, we examined the functional properties of vBNST-projecting, IC neurons in male and female mice that have undergone short-term ethanol exposure and abstinence using a voluntary Drinking in the Dark paradigm (DID) paired with whole-cell slice electrophysiology. First we show that IC neurons projected predominantly to the vBNST. Next, our data show that short-term ethanol exposure and abstinence enhanced excitatory synaptic strength onto vBNST-projecting, IC neurons in both sexes. However, we observed diametrically opposing modifications in excitability across sexes. In particular, short-term ethanol exposure resulted in increased intrinsic excitability of vBNST-projecting, IC neurons in females but not in males. Furthermore, in females, abstinence decreased the excitability of these same neurons. Taken together these findings show that short-term ethanol exposure, as well as the abstinence cause sex-related adaptations in BNST-projecting, IC neurons.
Keywords: BNST; Binge ethanol drinking; Electrophysiology; Insula; Sex difference.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures



References
-
- Alexander GM, Graef JD, Hammarback JA, Nordskog BK, Burnett EJ, Daunais JB, Bennett AJ, Friedman DP, Suomi SJ, & Godwin DW (2012). Disruptions in serotonergic regulation of cortical glutamate release in primate insular cortex in response to chronic ethanol and nursery rearing. Neuroscience, 207. 10.1016/j.neuroscience.2012.01.027 - DOI - PMC - PubMed
-
- Cannady R, Rinker JA, Nimitvilai S, Woodward JJ, & Mulholland PJ (2018). Erratum: Chronic alcohol, intrinsic excitability, and potassium channels: Neuroadaptations and drinking behavior (Handbook of Experimental Pharmacology, 10.1007/164_2017_90. In Handbook of Experimental Pharmacology (Vol. 248). 10.1007/164_2018_192 - DOI - DOI - PMC - PubMed
-
- Centanni SW, Morris BD, Luchsinger JR, Bedse G, Fetterly TL, Patel S, & Winder DG (2019). Endocannabinoid control of the insular-bed nucleus of the stria terminalis circuit regulates negative affective behavior associated with alcohol abstinence. Neuropsychopharmacology, 44(3). 10.1038/s41386-018-0257-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

