Loss of TRIM21 alleviates cardiotoxicity by suppressing ferroptosis induced by the chemotherapeutic agent doxorubicin
- PMID: 34233258
- PMCID: PMC8261003
- DOI: 10.1016/j.ebiom.2021.103456
Loss of TRIM21 alleviates cardiotoxicity by suppressing ferroptosis induced by the chemotherapeutic agent doxorubicin
Abstract
Background: Doxorubicin, an anthracycline chemotherapeutic agent, is widely used in the treatment of many cancers. However, doxorubicin posts a great risk of adverse cardiovascular events, which are thought to be caused by oxidative stress. We recently reported that the ubiquitin E3 ligase TRIM21 interacts and ubiquitylates p62 and negatively regulates the p62-Keap1-Nrf2 antioxidant pathway. Therefore, we sought to determine the role TRIM21 in cardiotoxicity induced by oxidative damage.
Methods: Using TRIM21 knockout mice, we examined the effects of TRIM21 on cardiotoxicity induced by two oxidative damage models: the doxorubicin treatment model and the Left Anterior Descending (LAD) model. We also explored the underlying mechanism by RNA-sequencing of the heart tissues, and by treating the mouse embryonic fibroblasts (MEFs), immortalized rat cardiomyocyte line H9c2, and immortalized human cardiomyocyte line AC16 with doxorubicin.
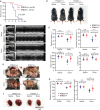
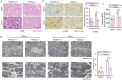
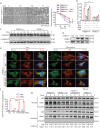
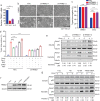
Findings: TRIM21 knockout mice are protected from heart failure and fatality in both the doxorubicin and LAD models. Hearts of doxorubicin-treated wild-type mice exhibit deformed mitochondria and elevated level of lipid peroxidation reminiscent of ferroptosis, which is alleviated in TRIM21 knockout hearts. Mechanistically, TRIM21-deficient heart tissues and cultured MEFs and H9c2 cells display enhanced p62 sequestration of Keap1 and are protected from doxorubicin-induced ferroptosis. Reconstitution of wild-type but not the E3 ligase-dead and the p62 binding-deficient TRIM21 mutants impedes the protection from doxorubicin-induced cell death.
Interpretation: Our study demonstrates that TRIM21 ablation protects doxorubicin-induced cardiotoxicity and illustrates a new function of TRIM21 in ferroptosis, and suggests TRIM21 as a therapeutic target for reducing chemotherapy-related cardiotoxicity.
Funding: NIH (CA129536; DK108989): data collection, analysis. Shanghai Pujiang Program (19PJ1401900): data collection. National Natural Science Foundation (31971161): data collection. Department of Veteran Affairs (BX004083): data collection. Tianjin Science and Technology Plan Project (17ZXMFSY00020): data collection.
Keywords: Antioxidant; Cardiotoxicity; Doxorubicin; Ferroptosis; TRIM21.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing interests.
Figures


Similar articles
-
The Ubiquitin E3 Ligase TRIM21 Promotes Hepatocarcinogenesis by Suppressing the p62-Keap1-Nrf2 Antioxidant Pathway.Cell Mol Gastroenterol Hepatol. 2021;11(5):1369-1385. doi: 10.1016/j.jcmgh.2021.01.007. Epub 2021 Jan 19. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33482392 Free PMC article.
-
Deubiquitinase MYSM1 promotes doxorubicin-induced cardiotoxicity by mediating TRIM21-ferroptosis axis in cardiomyocytes.Cell Commun Signal. 2024 Dec 18;22(1):593. doi: 10.1186/s12964-024-01955-6. Cell Commun Signal. 2024. PMID: 39695708 Free PMC article.
-
Qishen granule alleviates doxorubicin-induced cardiotoxicity by suppressing ferroptosis via nuclear erythroid factor 2-related factor 2 (Nrf2) pathway.J Ethnopharmacol. 2024 Dec 5;335:118604. doi: 10.1016/j.jep.2024.118604. Epub 2024 Jul 22. J Ethnopharmacol. 2024. PMID: 39047881
-
Therapeutic Targets for DOX-Induced Cardiomyopathy: Role of Apoptosis vs. Ferroptosis.Int J Mol Sci. 2022 Jan 26;23(3):1414. doi: 10.3390/ijms23031414. Int J Mol Sci. 2022. PMID: 35163335 Free PMC article. Review.
-
The role of ferroptosis in cardio-oncology.Arch Toxicol. 2024 Mar;98(3):709-734. doi: 10.1007/s00204-023-03665-3. Epub 2024 Jan 5. Arch Toxicol. 2024. PMID: 38182913 Review.
Cited by
-
Heat Shock Proteins and Ferroptosis.Front Cell Dev Biol. 2022 Apr 11;10:864635. doi: 10.3389/fcell.2022.864635. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35478955 Free PMC article. Review.
-
Nrf2: a dark horse in doxorubicin-induced cardiotoxicity.Cell Death Discov. 2023 Jul 26;9(1):261. doi: 10.1038/s41420-023-01565-0. Cell Death Discov. 2023. PMID: 37495572 Free PMC article. Review.
-
The initiator of neuroexcitotoxicity and ferroptosis in ischemic stroke: Glutamate accumulation.Front Mol Neurosci. 2023 Mar 23;16:1113081. doi: 10.3389/fnmol.2023.1113081. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37033381 Free PMC article. Review.
-
Role of oxidative stress and inflammation-related signaling pathways in doxorubicin-induced cardiomyopathy.Cell Commun Signal. 2023 Mar 14;21(1):61. doi: 10.1186/s12964-023-01077-5. Cell Commun Signal. 2023. PMID: 36918950 Free PMC article. Review.
-
Cardiomyocyte Atrophy, an Underestimated Contributor in Doxorubicin-Induced Cardiotoxicity.Front Cardiovasc Med. 2022 Feb 25;9:812578. doi: 10.3389/fcvm.2022.812578. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35282350 Free PMC article. Review.
References
-
- Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–170. - PubMed
-
- Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev. 2014;34(1):106–135. - PubMed
-
- Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104(12):971–977. - PubMed
-
- Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

