PD-L1 Testing in Cytological Non-Small Cell Lung Cancer Specimens: A Comparison with Biopsies and Review of the Literature
- PMID: 34233336
- PMCID: PMC8686721
- DOI: 10.1159/000517078
PD-L1 Testing in Cytological Non-Small Cell Lung Cancer Specimens: A Comparison with Biopsies and Review of the Literature
Abstract
Introduction: Programmed death-ligand 1 (PD-L1) expression is used for treatment prediction in non-small cell lung cancer (NSCLC). While cytology may be the only available material in the routine clinical setting, testing in clinical trials has mainly been based on biopsies.
Methods: We included 2 retrospective cohorts of paired, concurrently sampled, cytological specimens and biopsies. Also, the literature on PD-L1 in paired cytological/histological samples was reviewed. Focus was on the cutoff levels ≥1 and ≥50% positive tumor cells.
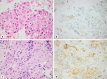
Results: Using a 3-tier scale, PD-L1 was concordant in 40/47 (85%) and 66/97 (68%) of the paired NSCLC cases in the 2 cohorts, with kappa 0.77 and 0.49, respectively. In the former cohort, all discordant cases had lower score in cytology. In both cohorts, concordance was lower in samples from different sites (e.g., biopsy from primary tumor and cytology from pleural effusion). Based on 25 published studies including about 1,700 paired cytology/histology cases, the median (range) concordance was 81-85% (62-100%) at cutoff 1% for a positive PD-L1 staining and 89% (67-100%) at cutoff 50%.
Conclusions: The overall concordance of PD-L1 between cytology and biopsies is rather good but with significant variation between laboratories, which calls for local quality assurance.
Keywords: 22C3; 28-8; Cell block; CytoLyt; Cytology; Histology; PreservCyt.
© 2021 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50. - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. - PubMed
-
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–30. - PubMed
-
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29. - PubMed
-
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

